Covid-19 Vaccine
Covid Vaccination Information and contraindications sheet.docx
Today the FDA released Pfizer’s Phase III vaccine trial data. This is big, as this is the first time we, scientists, have seen the data.
Things are looking good. Well, not just good, they are looking great. This trial was as clean as it can get. There is nothing (big) we didn't expect.
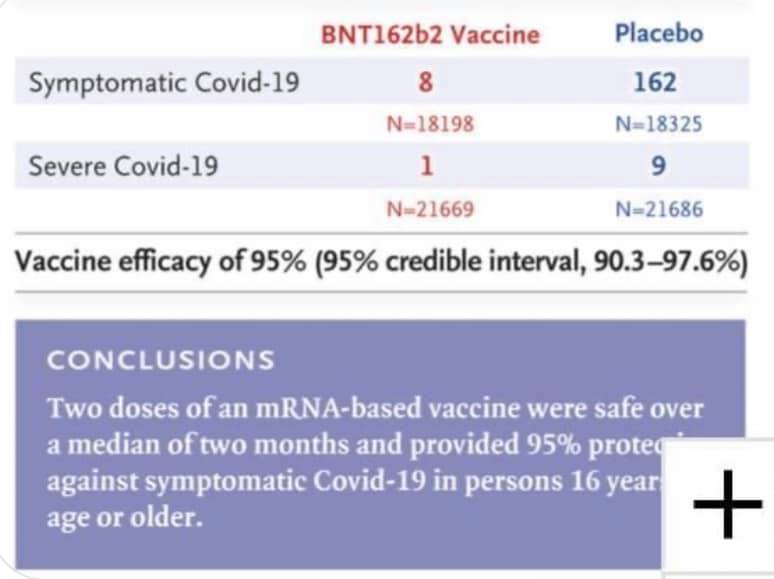
Efficacy. • We see benefit starting 14 days after the first dose (see Figure). This makes sense because it takes about 2 weeks for the immune system to make sufficient antibody protection after vaccination. • After the first dose, efficacy was 52%. This is a fantastic for a single dose. • After your second dose (3 weeks later), efficacy is 95%. We are confident that efficacy ranges from 90-98%. • Efficacy doesn’t meaningfully differ by age, race/ethnicity, or comorbidity.
Adverse events.
• Overall, the Pfizer vaccination gives more adverse events than the flu vaccination but less than the shingles vaccine.
• The most common solicited adverse reaction (i.e. what we were expecting) were mild to moderate: injection site reactions (84.1%), fatigue (62.9%), headache (55.1%), muscle pain (38.3%), chills (31.9%), joint pain (23.6%), fever (14.2%)
• The chance of getting a severe adverse event was low (0.5%). A severe event is more likely in the second dose than the first and were less frequent among older adults compared to younger participants.
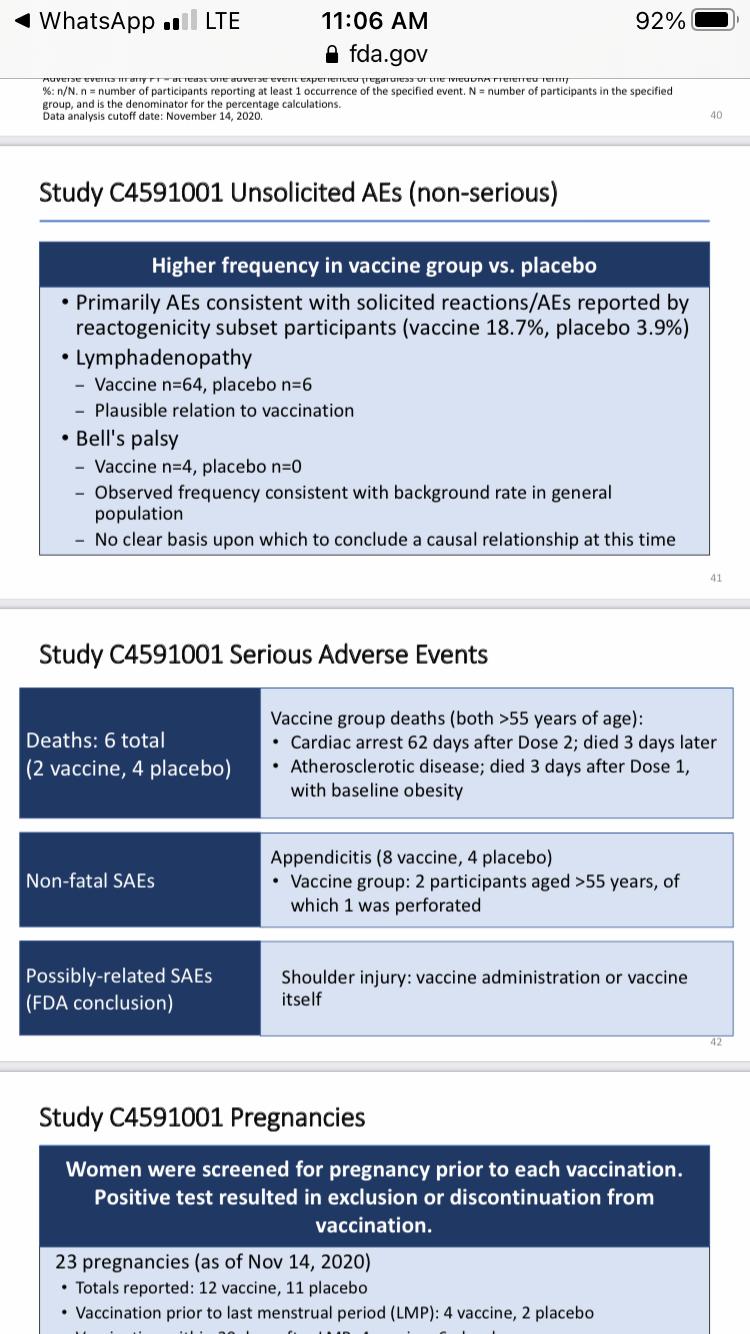
• An unsolicited (i.e. not expected) adverse event that came up more in the vaccine group was swollen lymph nodes (lymphadenopathy). 64 people in the vaccine group compared to 6 people in the placebo group. This could be related to the vaccine.
• There were 4 people in the vaccine group that got Bell’s palsy (compared to 0 in the placebo group). These cases occurred at 3,
9, 37, and 48 days after vaccination. This isn’t much of a concern because it reflects the same rate of Bell’s palsy in the general population. Something to keep an eye on
• 6 people died during the trial (4 in placebo; 2 in vaccine group). Deaths were NOT related to the vaccine. Mostly related to heart attacks.
There are a few unknowns. From this study, we do not know… • Duration of protection • Effectiveness against transmission • Effectiveness or safety among certain high-risk populations, such as children less than 16 years of age, pregnant and lactating individuals, and immunocompromised individuals. There were just not enough people in the study to make meaningful conclusions. This likely means that the FDA will not approve emergency use for these populations. We will know more on Thursday. • Benefits of individuals with prior COVID19 infection. But, given there is documented reinfection, the FDA stated that previously infected people could benefit from vaccination.

The Pfizer BioNTech COVID-19 Vaccine includes the following ingredients: mRNA, lipids ((4-hydroxybutyl)azanediyl)bis(hexane-6,1-diyl)bis(2-hexyldecanoate), 2 [(polyethylene glycol)-2000]-N,N-ditetradecylacetamide, 1,2-Distearoyl-sn-glycero-3- phosphocholine, and cholesterol), potassium chloride, monobasic potassium phosphate, sodium chloride, dibasic sodium phosphate dihydrate, and sucrose
VRBPAC-12.10.20-Meeting-Briefing-Document-FDA.pdf
VRBPAC-12.10.20-Meeting-Briefing-Document-Sponsor.pdf
https://vaccine.unchealthcare.org/science/the-science-of-mrna-vaccines/
https://onlinelibrary.wiley.com/doi/full/10.1002/art.41450
Safety and Efficacy of the BNT162b2 mRNA Covid-19 Vaccine
https://www.nejm.org/doi/full/10.1056/NEJMoa2034577?fbclid=IwAR1WjTT1OoxE6mJH2rUD8C01mo7TLeocw1H2eFMpuySrRUSOs0XnkvuciB8
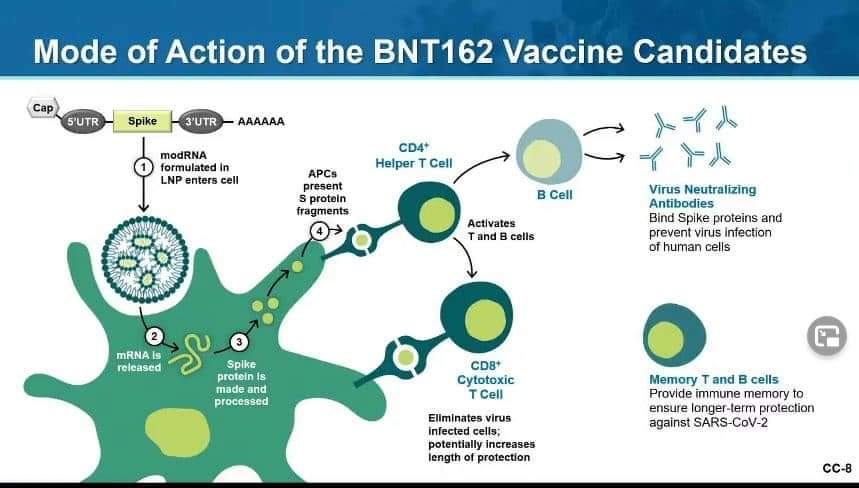
Q and A
Dr. Barney Graham, MD, Ph.D. is Deputy Director and Chief of the Viral Pathogenesis Laboratory at the National Institute of Allergy and Infectious Diseases Vaccine Research Center. He is an expert in vaccine development for viral diseases, viral pathogenesis, and mechanisms of immunity and pandemic preparedness. His laboratory helped develop the science for creation of mRNA coronavirus vaccines and was instrumental in the development of the Moderna vaccine (which just received EUA approval).
The Pfizer and Moderna vaccines are very similar. The goal is to have the body create an immune response against the coronavirus spike protein (which the virus uses to attach to the ACE2 receptor, creating a bridge for transfer of the viral genome to the host cell, and beginning the infection process). Both vaccines are mRNA vaccines, which are delivered in a lipid nanoparticle (which protects the mRNA long enough to have it enter the cells of the vaccinated person). Once inside the cell, the mRNA signals the cell to create a spike protein which it then pushes to the cell membrane surface. The body then generates antibodies directed against these foreign proteins. If the body were subsequently presented with the SARS CoV-2 virus, these antibodies would block the spike protein and thereby prevent infection.
The durability of the antibody response is very good (now out to six months in a vaccine study of participants, with little degradation) using the two-dose methodology. It appears that these vaccines will provide long-lasting immunity.
Both the Pfizer and Moderna vaccines have about 95% effectiveness (slightly lower in certain populations), which is extraordinary. All of the severe cases of COVID-19 infection (hospitalizations, etc.) in the Moderna trial occurred in the placebo group. This seems to indicate that even if you are vaccinated and still contract the virus, the infection is less severe.
Q: Can someone who receives the COVID-19 vaccine transmit the virus to a non-vaccinated person?
A: It’s too early to know. You will probably still be able to carry the virus in the upper respiratory track, but the viral load and the period of shedding will be shorter, which should lead to less transmission (much less likely to shed the virus).
Q: Will people be screened for serologic antibodies to COVID-19 prior to vaccination (revealing if someone previously had an unknown infection)?
A: Experts believe that the immunity created by the vaccination will be better than natural immunity that is created by getting the infection. Studies already seem to indicate that this is true. For this reason, even previously infected people are recommended to get vaccinated. Serology testing prior to vaccination is also a logistical challenge, so this is not likely.
Q: Can the mRNA enter the nucleus and somehow interfere with the host genome?
A: mRNA vaccines have been studied for 20 years. There is no evidence that the mRNA can enter the nucleus of the cell.
Q: What are the short-term vaccine side effects?
A: In the mRNA vaccines, most of the side effects (fever, chills, muscle aches, etc.) occurred after the second dose. They were generally well tolerated. Very few people needed to take Motrin or Tylenol, for example.
Q: Can the vaccine lead to long-term side effects?
A: We don’t know, but 98% of vaccine side effects in the studies generally occurred in the first six weeks after vaccination. We are at the six-month mark in the studies and no long-term side effects have been identified. We will learn more as the studies continue.
Q: What about anaphylactoid reactions?
A: There have been some anaphylactoid reactions reported. We don’t completely understand why this is happening (it is probably not an IGE mediated event). Perhaps they are due to mast cell degranulation. The recommendations around this will continue to change as we learn more.
Q: Can I get the vaccine if I am pregnant or planning to become pregnant?
A: Pregnant patients were not enrolled in the studies. However, some participants became pregnant and there have been no adverse outcomes to date. Reproductive toxicity studies also had no adverse findings. This issue will be addressed in future trials and studies. The general feeling is that these vaccines will be safe for pregnant and breastfeeding women, but we don’t have a lot of data on this yet. The decision to vaccinate or not will require an individual discussion with your obstetrician.
Q: Can various types of vaccines be given to the same person? In other words, if I received the Pfizer vaccine as a first dose, can I get the Moderna vaccine for the second dose or vice versa?
A: The recommendation is that you take your second dose from the same manufacturer. This is the way both vaccines were studied and tested.
Q: Should I be concerned that if I have received a first dose, that there may not be enough vaccine to receive a second dose?
A: There is a second dose saved for every individual who has received a first dose, so you are guaranteed to receive both.
Q: How does the vaccine distribution work?
A: The vaccine is being distributed based on population to 64 jurisdictions (50 states, 6 territories, 8 large cities). Each jurisdiction then makes its own logical distribution plans. The CDC recommendation is that they be divided between healthcare facilities and nursing home facilities. The Pfizer vaccine was distributed initially to larger hospitals because they have the resources to maintain the vaccine at the required -80° F temperature. It will become more widely available over time. The distribution plan is evolving as we speak.
Q: When can we get to a point where an organization like NAPA can purchase and distribute vaccine (currently not possible due to access restraints, distribution controls, storage limitations, etc.)?
A: An mRNA vaccination strategy has never scaled up prior to 2020. The development of the vaccine and the scale-up of the complex distribution logistics have occurred simultaneously. The national distribution strategy and logistical infrastructure will evolve over the next few months.
https://www.youtube.com/watch?v=4e_9H7MQuuM&feature=youtu.be
Q and A
Some questions and answers copied/pasted from other professionals as these make good sense:
Q How would they control which cells take up the mRNA?
Ultimately, you would end up with spike proteins expressed on some cell membranes and circulating anti-spike protein antibodies. If the antibodies attach to the spike proteins on the cell membranes and fix complement leading to the formation of a MAC (membrane attack complex) or if the cell is attacked by a killer T-cell then the cell will be lysed. How do they ensure that neurons and myocardiocytes do NOT express the spike proteins? And if they can’t then it’s inevitable that there will be a minimal degree of “autoimmunity” following the vaccination. Of course that’s not going to be permanent because only a fraction of cells will take up the mRNA which will degrade rather quickly. Is the bet that the amount of cells is big enough to induce immunity but small enough to avoid any clinically detectable autoimmune side-effects? Obviously, the trials so far seem to indicate safety.
A The vaccine is IM and won't disseminate much beyond the local injection site. Expression is transient as you said, but in effect this is why vaccines hurt more than placebo - there's the local immune response. And yes, we would have seen this years ago in early mRNA work as well as the clinical trials. The lack of dissemination is a weakness of this tech as regards things like gene therapy (where they have preferred to try retroviral vectors delivered by infusion) but an advantage when it comes to immunization.
The MRNA is a punch card. It will be delivered Intramuscular to the Deltoids Myocyte where the sars covid spike protein will be produced locally by the ribosome.
This will induce a immune reaction and produce antibodies to the sars covid spike protein LOCALLY.
Without alterations to the genome DNA (in the nucleus of the cell) there is NO WAY other cells will be able to produce the spike protein as they won’t be able to produce more MRNA (dont have the instructions).
The vaccine mrna will degrade within hours locally.
Normally companies produce the protein and inject it. The production of the protein is more complex for which smart scientists decided to use our cellular mechanisms. This makes the vaccine more vulnerable to degradation (Needs low temp) but much more easier to produce.
As written above, this is a very transient response - the mRNA is degraded locally within a few hours, whereas it takes weeks to generate an antibody response. Therefore, the mRNA (and therefore protein) will be long gone before any antibodies are present.
So what happens if you were exposed to SARS-CoV-2 already and had antibodies directed against it? Again, with such a local isolation of the cells actually making the targeted protein, you shouldn't really have an exuberant systemic immune response, and the mRNA/proteins are so short-lived anyway.
In several clinical trials with around 30,000 per trial, if there was an autoimmune inducing component to these vaccines, we should have seen a signal (given the prevalence of autoimmunity and people with risk factors for autoimmunity).
There is a theoretical risk with mRNA vaccines for AI diseases; it is noted that some platforms create a fairly profound Type I interferon response, and that raises the concern that it may cause exacerbations of existing autoimmune disease, like RA/SLE (mRNA is a PAMP, so it can activate Toll-like receptors causing a cascade leading to the Type I interferon release). That being said, there are some ways to mitigate this; Pfizer has incorporated one such way, with modification of their mRNA to use pseudouridine instead of uridine, dampening down this effect.
In practical use, there hasn't been any reports of increased RA/SLE flares that I've seen. In the informal groups, I've seen reports that some have flares, but they attribute that to the fact they've been off their DMARDs due to COVID. I think the quote was "Yes, it's a flare, but it's far from the worst flare I've had." Also, the data in the interim Phase 3 report / FDA analysis doesn't make any note of increased autoimmune disease activity in the vaccine arm.
The risk that COVID poses to patients with lupus is profound; according to a study in 224 SLE patients with 41 positive for COVID, 24 (60%!) needed to be hospitalized, 4 in the ICU, and 4 died. That's 10% critical care use, and 10% mortality, which is far worse than what we expect in the general population. I would indeed recommend the vaccine to this population, all other things being equal.
Reference for lupus and COVID: https://onlinelibrary.wiley.com/doi/full/10.1002/art.41450
Along those same lines of thinking though, Moderna does not have the same uridine modification Pfizer does. This is probably why it generates so much more in the way of antibodies, but theoretically may increase the risk of AI flare. If availability were equal, I would probably suggest Pfizer over Moderna for AI patients.
Here is the explanation from Paul Offit, vaccinologist at Penn who is on the advisory board for the FDA (excerpt is from recent UCSF Covid Grand Rounds):
Q: You talked the lack of concern about a significant long term side effect emerging after the two months, what about the fact that these are mRNA vaccines, which is a new way of doing vaccines? A colleague of mine wrote me the other day, “Is there any concern that these things are going to continue to churn out proteins that are going to cause a low level inflammatory response, or just something weird we haven’t seen before because this is a brand new vaccine technology?
A: This is a new technology and therefore there are unknowns. I think you have to be humble and keep your eyes open and realize there may be things over the next year or two that we hadn’t anticipated on happening. I came into the research world studying rotavirus. By the time the first rotavirus vaccine came out that virus had been studied in experimental animals and people for 50 years. After it came out it was found to be a rare cause of intussusception (intestinal blockage). That was not predicted by animal models and it was not clear at all that natural human rotavirus caused intussusception. that surprise came after 50 years of studies so you have to be humble that there may be things that you don’t know. I do think that there systems in place like the Vaccine Adverse Events Reporting System, the Vaccine Data and Safety link, The V-Safe System, the CSA System that is opening its doors the minute this vaccine rolls out to see whether there’s a problem. Remember you make messenger RNA all the time, you’re making proteins all the time. It’s a very labile molecule, it tends to break down quickly so I don’t really worry about that. What people say to me is “Yeah, but won’t it incorporate itself into your DNA and cause you to have a variety of adverse consequences?” No – it's mRNA and not DNA. It only enters the cytoplasm. It doesn’t enter the nucleus. There are DNA vaccines - in order to the get the DNA to get into the nucleus where it needs to go, you have to use an electroporation device, which is essentially an electric shock which makes the cells more porous which allows the DNA to get in there. Although I never understand why it is that people are always worried that you’re going to change your DNA to have autism or diabetes. Nobody ever thinks it’s going to change your DNA to become like spiderman or have x-ray vision. Both are as likely actually.
While the concept seems simple, it required decades of work for mRNA vaccines to overcome a series of hurdles. First, scientists learned how to modify mRNA so that it did not produce violent immune system reactions. Second, they learned how to encourage immune system cells to gobble up the mRNA as it passed by in the blood. Third, they learned how to coax those cells to make large amounts of the critical piece of protein. Finally, they learned how to enclose the mRNA inside microscopically small capsules to protect it from being destroyed by chemicals in our blood.
Along the way, they also learned that, compared to traditional vaccines, mRNA vaccines can actually generate a stronger type of immunity: they stimulate the immune system to make antibodies and immune system killer cells — a double strike at the virus
Q. Would the Pfizer Covid vaccine make you positive for the Covid PCR test?
A. No.
No way, but would make you positive for ELISA for spike protein.
No because PCR doesn't test for antibodies or immune response. Iit tests for antigens.
The Abott ID tests for the nucelocapsid protein not the spike.
Not the pcr test, but some of the ag tests are based on the spike protein, so those tests will be positive. Have to chose the right ag test if using one.
No. The vaccine will make u produce antibodies against the spike protein. The real time PCR is a test to determine the presence of the virus, not your immune response.
Q. Can we please talk about the relative contraindication of getting vaccinated if you were covid + within 90 days. Would you wait?
A. It’s not a relative contraindication. The recommendation is for hcw to “consider” waiting 90 days after infection due to supply issues.
Q. What are the facts on re-infection from Covid? Since I have Covid, do I need the vaccine?
A. No stats yet on re-infection, except we know it’s possible and is occurring. So, yes to vaccine 90 days post COVID.
Reinfection rare but possible after 3 months and yes you need vaccine.
CDC recommend vaccine 90 days after infection. Reinfection rate is really not known.
There is reinfectionm, though rare and usually after 90 days of prior infx. And yes, u can get the vaccine if u had covid in the past. The 90 d pre vaccine applies in places of short supply. CDC recommends to wait 90 d prior to vaccine if u received monoclona Ab or convalescent plasma as tx for covid.
The latest update from CDC was that you’re eligible for vaccination as early as 14 days post infection, but may elect to wait up to 90 days as reinfection is rare and unlikely in that timeframe. However, there’s some data to suggest that variation of strains or degree of exposure may play a role, and vaccination may protect better than natural immunity. If you’ve had convalescent plasma or monoclonal antibody treatment, you must wait 90 days.
Q. If your hospital gives you an option to choose, which one would you get, pfizer or moderna and what would your reasoning be for that choice? I'm seeing articles with pfizer causing more side effects than that for moderna but not sure if that is due to pfizer being administered more than moderna or actual more side effects.
A. In terms of mechanism of action and overall design these are extremely similar.
Moderna doesn’t have to be at certain temps? If that’s the case then I would choose that one due to lack of possible errors with temps being maintained with Pfizer. Their studies included more demographically diverse people and had no severe infections in their vaccinated arm. It seems their vaccine can be stored more easily as well.
Pfizer has a pretty impressive set up with GPS and temperature tracking devices that will alarm if the vaccines go above a certain temp, etc.
The temperature standard are scary. There are so many rules for Pfizer. You have to thaw it, but you can’t refreeze it.
If J&J and AZ Adenovirus vector vaccine trials are demonstrated to be reasonably safe, I can consider them. But do not know why some people are MORE leery of mRNA vaccine than Ad vector vaccines.
mRNA is simply not replicated in human cells and doesn’t last.
On the other hand, in Ad vector vaccine, the neutered Ad vector inserts a DNA transgene which becomes episomal DNA plasmid in your nucleus, which lasts much longer than mRNA.
In fact your cells transcribe the inserted episome DNA to make mRNA which is translated to spike protein.
So I don’t see how some people consider having replicating episomal DNA (that makes mRNA) inserted into their cells less of a concern than getting mRNA in their cells.
In addition if you have prior exposure to the Adenovirus vector they use, your immune system may just stop the vaccine from ever working at all. That’s why in some countries the J&J and AZ vaccines may be very ineffective.
“Conversely, the main disadvantage compared to integrating vectors is that the AdV DNA vector persists as an episome in the trans- duced cells. Thus, transgene expression is tran- sient and particularly vulnerable to cell silencing mechanisms. Finally, the episome is diluted by duplication of transduced cells. However, lack of integration, as discussed below, is also an advan- tage as it increases the safety profile. AdV vectors find their primary application in vaccination and clinical trials in which transient rather than sta- ble expression of the transgene is pursued.”
https://www.researchgate.net/.../Viral-vectors-A-look...
I think pfizer/biontechs had a severe case in the vaccine group but modernas did not. Might just be chance but ive been keeping it in the back of my mind. Also modernas dose is higher than biontechs for those that are wary of mrna for whatever reason. I would think mrna would be better than adenovirus vectored since it stays in the cytoplasm of the cells and doesn't cross into the nucleus.
Any thoughts about the dilution problem with Pfizer vaccine? I see report says there's extra dose left in the vial(maybe due to dilutional error or waste protection?)and FDA OK to use. Will taking this "extra" dose leads to lower dosing and cause the vaccine being less effective?
In this case, will Moderna be a better choice since it doesn't need to be diluted?
Pfizer. You will get the second dose and be done in 3 weeks instead of 4. There really is no comparative data. It is also interesting to observe how much logistical effort goes into thawing, drawing up the Pfizer vaccine and making sure the subjects are lined up and ready.
Two (Moderna/Pfizer) are mRNA, the other (Astra-Z) is... a protein-the 'rona spike protein, modified.
Q. Can you get COVID-19 from mRNA vaccines?
A. No
Q. Can mRNA vaccine result in positive COVID PCR?
A. No.
Q. Do you need to get COVID PCR or serology before getting vaccine?
A. No
Q. Should you keep wearing mask after vaccine?
A. Yes.
We don’t know yet how good is vaccine in preventing asymptomatic infection and transmission. So until we have such data.
Q. How long the side effects of vaccine last?
A. They usually start within 24 hours and last < 2days.
Q. How do you tell the difference between vaccine side effects and COVID symptoms?
A. Typically vaccine side effects include headache, fever, fatigue, and muscle aches. Vaccine does not cause respiratory or GI symptoms. So if you have cough, shortness of breath, runny nose, sore throat, or loss of taste and smell, get yourself checked for COVID.
Q. What if I can’t get 2nd vaccine shot on day 21 (for Pfizer) or day 24 (for Moderna)?
A. Don’t worry. These are minimum time frames. There is no harm if 2nd dose gets delayed due to some reason. Get the second shot when you can. (No need to repeat the first dose).
Q. Can I mix and match vaccines?
A. No, you shouldn’t. It might work but this is not the time to do experiments on your own.I just had flu shot, how long should I wait to get COVID vaccine? 14 days.
Q. I previously had COVID, should I still get a shot?
A. There is no anticipated harm. Immunity from natural infection may wane after few months. Perhaps best to wait 2 to 3 months from date of COVID infection in my opinion. We will have more data soon.
It will further boost ur immunity. Covid antibodies do not last long.
Take when you feel 100% back to normal and have not been on steroids.
Wait for 3 months, You already have immunity.
You can get it now if asymptomatic or wait. I think they were recommending delaying it due to vaccine supply issues but if your institution doesn’t have that problem then would get it.
It is not an absolute contraindication according to Pfizer data on its website. However you are expected to have some immunity likely over the next 90 days and it is recommended that you wait so others without protection can get the vaccine.
It does not make sense to get the vaccine if you have had COVID infection and have the antibodies. Immunologically, your body would make antibodies to multiple proteins of the virus and therefore it would be a more complete immunity as opposed to an immunity from a vaccine which only has a single spike protein. As for the argument regarding immunity fading, again there is hardly any reliable data to support that claim and there is no data that similar would not happen with the vaccine.
If you have antibodies, there is no evidence that you need vaccination. I would not, and didn’t, for that reason. The CDC says you should, but that is based on no objective data that I’ve seen showing that antibodies are superior from one over the other. We know that antibodies fade after some mild infections, but we don’t know if they also do after a period after vaccination. We also don’t know if retained memory cells ultimately confer similar immunity. We just don’t know, but recent studies seem to suggest that early immunity after natural infection is quite robust.
One study suggests higher average antibodies titers in vaccine groups. This is from early trial data. It doesn’t reflect the average age and comorbidity of those being vaccinated. It doesn’t prove that those with high titers from natural infection benefit, and it doesn’t prove that higher antibody titers yield resistance that is superior.
Vaccinating anyone who had a prior infection at this point is a waste. Use the limited doses on those with no immunity. That gets us to herd immunity more quickly if it works.
While some reports have antibodies by 6 mos, some show 50% decrease by 3 mos; the less the infection, the less the immune response.
You are protected against the strain you received for a few months. The vaccine would provide wider coverage for other strains (not sure of the new variant).
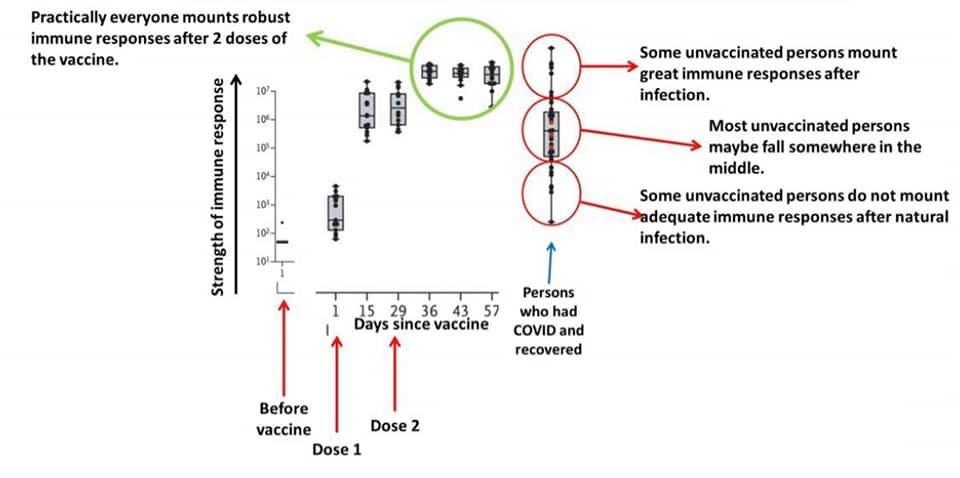

Q. How long patients who received convalescent plasma or monoclonal antibodies wait before getting shot?
A. 90 days.
Q. Can immunocompromised patients get COVID vaccine?
A. We don’t know how well the vaccine works in these patients but there is no harm. So Yes.
Q. What if there is a reaction after getting vaccine? A. Check tryptase level. Tryptase is a mast cell marker that is elevated in true anaphylaxis. Elevated levels will help weed out true anaphylaxis. The result is more important for decision later regarding was this anaphylaxis, what should we do for second dose. Acute reaction will already have been managed by then.
Q. Is it safe for pregnant and breastfeeding women to take the vaccine? A. No data yet. But it’s not live vaccine. So no anticipated special risk in pregnancy. Talk to your patients and let them decide.
mRNA is very short lived and is degraded intracellularly soon after it is translated into protein. It will not spread systemically to breast milk glands and is not secreted from cells into milk. Hypothetically speaking even if baby swallows some mRNA, it gets degraded rapidly in gi tract (we are all on a daily basis inhaling and ingesting lots of viruses and their genetic material and our gi system is filled with enzymes and acids to degrade them).
The spike protein made from vaccine will last on the antigen presenting cells for their lifespan, which is a few days. Again, it is not going to spread systemically (what the immune system is going to do is contain it locally, coz it thinks this is an infection and doesn’t want it to spread). Mom’s antigen presenting cells don’t cross placenta. Again, hypothetically speaking if a baby were to swallow some spike protein, it will get digested just like all proteins get digested down to amino acids in gi tract.
Antigen presenting cells do not cross placenta, theoretically this means that it is safe for pregnant females.
mrRNA is degraded within hours within the muscle it was injected and the spike protein it then causes our cells to make cannot physically pass through the placenta (it’s 180-200 kDa, the same size as IgM which also can’t pass through placenta). However, we DO know all the bad things that can happen to pregnant women who get covid- higher death and icu rates - and to their babies- risk of preterm and stillbirth as well as IUGR.
Q. Why is it being recommended that people get a vaccine for Covid after already having an infection?
In the Moderna trial, there were 30,000 participants with half being in the vaccine group and 11 people getting infected after vaccine. In the Pfizer trial there was 43,000 people with half the participants being in the vaccine group, with 8 infections. There has been only a handful of reported reinfections after Covid Infection. There could be an under reporting of reinfections, but with millions of people having Covid and the small number of reinfections, the efficacy of Covid infection reducing risk of reinfection would be a guess, 99.999 percent efficacy, higher than any of the data from the two main trials.
Some people do not develp antibodies even after infection. The IgG antibodies can disappear but you can still have immunity because it’s T-cell mediated.
Our best data showed that protective immunity from natural infection wanes (as in, most participants had more than a 50% reduction in protective neutralizing antibody) by 3 months. We don't have data yet about how well we develop effective memory B cells after natural infection. So, sure, you could gamble and say natural infection is enough, but saying it is "99.999% efficacy" is a baseless claim that is more likely to be untrue.
It is baseless, though. Your "99.999% efficacy" figure doesn't take into account that neutralizing antibody level drops rapidly (much faster than in those who were vaccinated), that not all people naturally infected produce any antibody at all, or that of those who do produce antibody it isn't guaranteed to have targeted a highly immuogenic portion of the spike protein, amongst other factors.
The vaccine appears to provide more lasting immunity than infection does perhaps because it generates a better immune response to the COVID spike protein than infection does.
Generally speaking natural infection generates a better immune response than vaccines. So I would be highly suspicious of such a claim until there is good evidence to back it up.
but how much better? Natural immunity to many coronaviruses appears to be only about 3 months in duration. Even if this is “better”, May only be, say, a year (which isn’t half bad given that we do flu vaccines each year)
The best “vaccine” is getting the actual disease, as no vaccine is able to stimulate the immune system in all the different ways a real infection does
that assumption is actually incorrect. The vaccine provides “better” immunity. The virus has many other confounding effects on the immune system.
https://news.yahoo.com/natural-immunity-covid-better-vaccine-163042399.html
Q. Can you get epidural steroid injection after getting vaccine?
A. There is no data on how well the vaccine works in immune compromised people. No one is sure what steroid dose interferes with antibody formation for this vaccine. The vaccine is likely not to work as well in immune compromised people based on data from previous vaccines.
It is local injection but still there is systemic absorption.
Delay the epidural as there is no reson to decrease vaccine efficiency.
There is no reason to risk the vaccine having less efficacy for a purely elective procedure.
CDC: Steroid therapy usually does not contraindicate administration of live-virus vaccines when such therapy is short term (less than 2 weeks); low to moderate dose; long-term, alternate-day treatment with short-acting preparations; maintenance physiologic doses (replacement therapy); or administered topically (skin or eyes), by aerosol, or by intra-articular, bursal, or tendon injection. The exact amount of systemic corticosteroids and the duration of their administration needed to suppress the immune system of an otherwise healthy child are not well defined. The immunosuppressive effects of steroid treatment vary, but many clinicians consider a dose equivalent to either 2 mg/kg of body weight or a total of 20 mg/day of prednisone as sufficiently immunosuppressive to raise concern about the safety of immunization with live-virus vaccines. Corticosteroids used in greater than physiologic doses also may reduce the immune response to vaccines. Physicians should wait at least 3 months after discontinuation of therapy before administering a live-virus vaccine to patients who have received high-dose, systemic steroids for greater than or equal to 2 weeks.
Q. Is it plausible that some of the reinfections could be because the first time infected was really a false positive? The pt was sick from a virus but not COVID-19? I was just wondering outloud how we can truly ever track reinfections unless the person gets retested through the same center? (basically I'm just wondering how we can compare an unknown if we don't have a mechanism to track it which it seems that is where we are...the same issue will come up with the vaccine as there still will be some people who get the disease)
The different strains aren’t different like flu strains. They are just different enough on a nucleotide level that we can tell them apart. That’s how they proved reinfections. Different sequencing.
Since the spike protein is what the vaccine is to and there have been no major changes in the spike protein the vaccine should stay the same.
Q. Anybody worried about COVID vaccine causing a autoimmune response or proliferative disorder? With a mRNA vaccine it seems like a possibility. Many of my doctor friends are trying to talk me out of getting it since I’m relatively young and healthy with no cormidibities. They say why risk it. Thoughts?
mRNA vaccines are less likely to do this than any real viral infection.
Why people are so insistent that mRNA vaccines are somehow more risky than...well...anything else. Our cells transcribe tens of thousands of mRNA transcripts daily, plus whatever our microbiome creates. For a small subset of people with reactogenicity issues the equation (certain exposure, uncertain risk versus uncertain exposure certain risk) needs to be considered I agree, but if anything it's looking like that may be more to do with ingredients other than the mRNA.
~5% of the population having had documented infection and the rate is 🚀 right now. A large percentage of the population plans to refuse the vaccine. The virus has multiple immunogenic proteins. I'd wager than the average exposure to immunogenic proteins from covid-19 will be at least as high in the non-vaccinated group as in the vaccinated group unless there is rapid, mass acceptance of these vaccines. It's also very clear that covid-19 has left significant disability in it's wake, even for people who were young and health... the vaccine not so much so far.
Our bodies deal with mRNA all of the time, mainly in the form of viral pathogens. The spike is foreign and would still be recognized as foreign. Immune response is to the foreign protein.
There have been multiple prior studies on DNA vaccines, which would be more of an autoimmune concern theoretically, in which they tracked anti-DNAase and have not ever seen autoantibody induced.
Think about the phase of viral assembly. Your ribosomes have made the proteins coded by the mRNA. They assemble to form additional virions, so they are making each protein including spike. The rna vaccine only has the mRNA for spike, so there is no reason to think there is a higher risk than when the virus is in the cell with all materials.
Why would a single spike protein be more at risk for autoimmunogenicity than the production of an entire virus?
What's your risk of getting covid? You need to think about that before knowing how to balance the risks and benefits.
If you have something autoimmune, covid will find it and destroy you.
Patients with autoimmune diseases on disease modifiers (DMARD) are recommended to take the vaccine, many take methotrexate and recommendations to hold it for 2 weeks especially for the first dose , not needed for second vaccine dose, as far as Pfizer and Modern Vaccines.
All Rheumatology Biologics are no concerns except Rituxan or B cell deleters , recommended To get the vaccine 2-3 weeks before and at least 2 months after the infusion.
Hold the methotrexate two weeks after the vaccine , steroids small doses (less than 10mg prednisone or equivalent) should be no concerns, high doses no clear guidelines but response to vaccine likely weakened but still would give two doses.
Xeljanz-----since it has more specific MOA , mild diminished response can be expected to happen to the vaccine but overall they usually build good immunogenicity to vaccines in general so no need to hold but these patients vary in disease activity and if it’s well controlled I’d like to hold it as well for 1-2 weeks, that’s the crucial time to respond to the vaccine.
No need to hold Humira.
Leukocytoclastic vasculitis after vaccination---- it’s usually self limited and would premedicate prior to next dose with H-1 , H-2 inhibitors and steroids small doses post injection if needed.
No concerns with Stelara and Xolair.
If possible to reduce the prednisone dose to 10 mg or less for the two weeks post first dose. Most Crucial time for vaccine response.
Should plaquenil be held? No. plaquenil has no immunosuppressive effects.
MTX is a once weekly dose, so if possible schedule the vaccine few days apart from last MTX dose and then two weeks off the medication.
No concerns regarding Orencia.
What about Cellcept? No concerns.
Cosentyx--continue the Cosentyx , as IL-17 blocker no concerns about the vaccine response.
Ixekizumab/Taltz or Cosentyx no concerns with the vaccine.
Entyvio is very selective for the gut and not an issue with vaccines.
It should be no concerns about the vaccine with IVIG
Q. Which vaccine use embyonic stem cells? Does any vaccine derive lipid particles from animal sources?
Neither the Pfizer nor the Moderna vaccine involved the use of cell lines that originated in fetal tissue taken from the body of an aborted baby at any level of design, development or production," the two prelates said. "They are not completely free from any connection to abortion, however, as both Pfizer and Moderna made use of a tainted cell line for one of the confirmatory lab tests of their products.
All of these COVID-19 vaccine developing companies used research that was conducted with embryonic cells lines. Virus won't replicate without cell lines and without this knowledge no one would be able to create a vaccine. It's disingenuous to claim that Pfizer and Moderna didn't create the vaccine with the help of those lines.
most cells lines are not derived from embryonic cells - it's perfectly possible to do research with human cells that aren't embryonic in origin.
ethical origin of all of these cell lines can be questioned. Look up origin of HeLa lines and tell me they were obtained with a consent. The black lady with cervical cancer never knew these famous cells would be experimented on at John Hopkins.
Instead of saying some vaccines are good, and some are bad based on what cell line they were researched and developed with, I would prefer to say all are good, because they are developed to save human lives.
why do you think that Moderna and Pfizer needed cell lines to generate mRNA vaccines? If you go back enough generations of research, cell lines were used at some point. Are religious objections going to get you to tell patients to avoid vaccines if Pfizer and Moderna used cell lines years ago but no cells are present in the final products?
as long as you aren’t aborting fetuses (that are otherwise viable) for the sole purpose of research or therapeutics, then it is permissible as long as you have permission from the parents.
“It is permissible to use stem cells for either legitimate scientific research or for therapy as long as its sources are legitimate, for example, adults if they give permission as long as it does not inflict harm on them; children with their guardian’s permission for a legal benefit without inflicting harm on them; placenta or umbilical cord blood with the permission of the parents; spontaneously aborted embryos or those aborted for a legally acceptable cause and with the permission of the parents; excess fertilised eggs [embryos] produced during the course of IVF and donated by the parents with assurance that they are not to be used to produce an illegal pregnancy. It is forbidden to obtain or use stem cells if the source is illegitimate as, for example, intentionally aborted foetuses (abortion without a legal medical reason); intentional fertilisation between a donated ovum and sperm; and therapeutic cloning” [25, p. 132).”
https://www.longdom.org/.../human-embryonic-stem-cell...
Q. For those who had covid with loss of smell and taste. How long did it take for taste and smell to at least start to return? How long til back to normal/close?
A. 1 to 8 weeks.
The first two weeks is the worst. Taste gradually came back over several weeks. Smell is still coming back slowly, I’m 8 weeks out.
I’m 9 weeks out and still not back to same. Can taste basics -salt/sweet and spicy but flavor still lacking a lot.
Widely different across different individuals. I did not get a covid test but had fever in late march. I still get a phantom tobacco smell once in a while that is triggered by strong tastes or smells. I came across one patient with the same symptoms.
6 weeks out. And smell is minimal still.
A friend mentioned smell training so I’ve tried that with smelling my candles. I think once smell comes back it’ll be better. spoke to an ENT who mentioned that and also Flonase daily. There are some case reports in the literature of using intranasal theophylline with anosmia. You can get it compounded. Because once you hit about 6 months it can become permanent and I’m worried about post viral permanent anosmia. It’s a small amount of theophylline and because it’s intranasal you don’t really get any systemic absorption so no need to have to check levels like you do for regular theophylline. olfactory rehab.
My nephew in his 20s got it 9 months ago... loss of smell only sx... says 90% back.
The first 6 weeks were terrible, but did eventually get better.
I was back to about 75% in 2 weeks. Took another month for me to get to 100%.
Only lost taste a couple of days. Smell took months to come back to baseline. But I never had a great sense of smell to begin with.
5 days both of mine were back to completely normal. My husband is 90 days out and smell still isn’t back to normal.
One of my friends is 6 months out and still has decreased smell.
My BIL lost smell 3 days in. Did neti pot TID (sterile solution of course) and it came back within 2 days.
Phantom tobacco and urine smell, it's really unpleasant. Outdoors in fresh air helps.
Usually comes back initially as dysosmia.
I’m 6 weeks out. It took 4 weeks for me to taste/smell anything, but it’s still very blunted. I can really only taste unprocessed foods like fruit and vegetables. Anything heavily processed doesn’t taste like much.
a lot of our nursing home and home COVID deaths have not been due to the respiratory issues, but have been elderly patients (often with dementia or some cognitive issues) who lost their sense of taste and smell and just dwindled over 1-2 months because they ate so poorly.
It took me 4-6 weeks, could tell sweet vs spicy at first. Definitely contributed to fatigue of COVID with anorexia. Have new food aversions now.
Might not come back.
Q. What is the route of vaccine administration?
A. Intramuscular.
Previous attempts with mRNA based approaches showed improved uptake with intradermal injection which would make sense as the skin has extensive dendritic cells to process. My guess is they went with IM since it is the easiest to replicate in large numbers.
I found it interesting to hear that most centers choose a one inch needle. I was always taught that the position of the muscle should be constant and your needle length should change specifically by weight as a guideline so someone less than 130lbs can have a 5/8” needle. 130-152 lbs a 1 inch needle, Between 152 and 200 for woman and 152 and 260bs for men you can choose a one inch or 1.5 inch and above 200lbs for Women and 260 lbs for men it has to be a 1.5 inch. The muscle should be brought to you so to speak as it risks subQ injection.
I just don’t see that kind of protocol or consideration or training being universally rolled out for their injectors.
There may be a chance that some of the non-responders or side effects were from subQ injections.
Q. What if you test positive for Covid after vaccination and are symptomatic?
A. Get monoclonal antibodies if you qualify i.e. with in 10 days of infection, age over 65, age over 55 with major comorbidities.
If you want the vaccine to work I would avoid the antibodies.
Q. If someone receives the vaccine, then gets covid a couple of weeks later, when should they get their second dose? I know the recommendations for the first dose were to delay vaccination until you recovered, but is that also the recommendation for the second dose?
A. Yes, you delay 2nd dose until you recovered.
Q. What are the side effects of vaccine?
A. 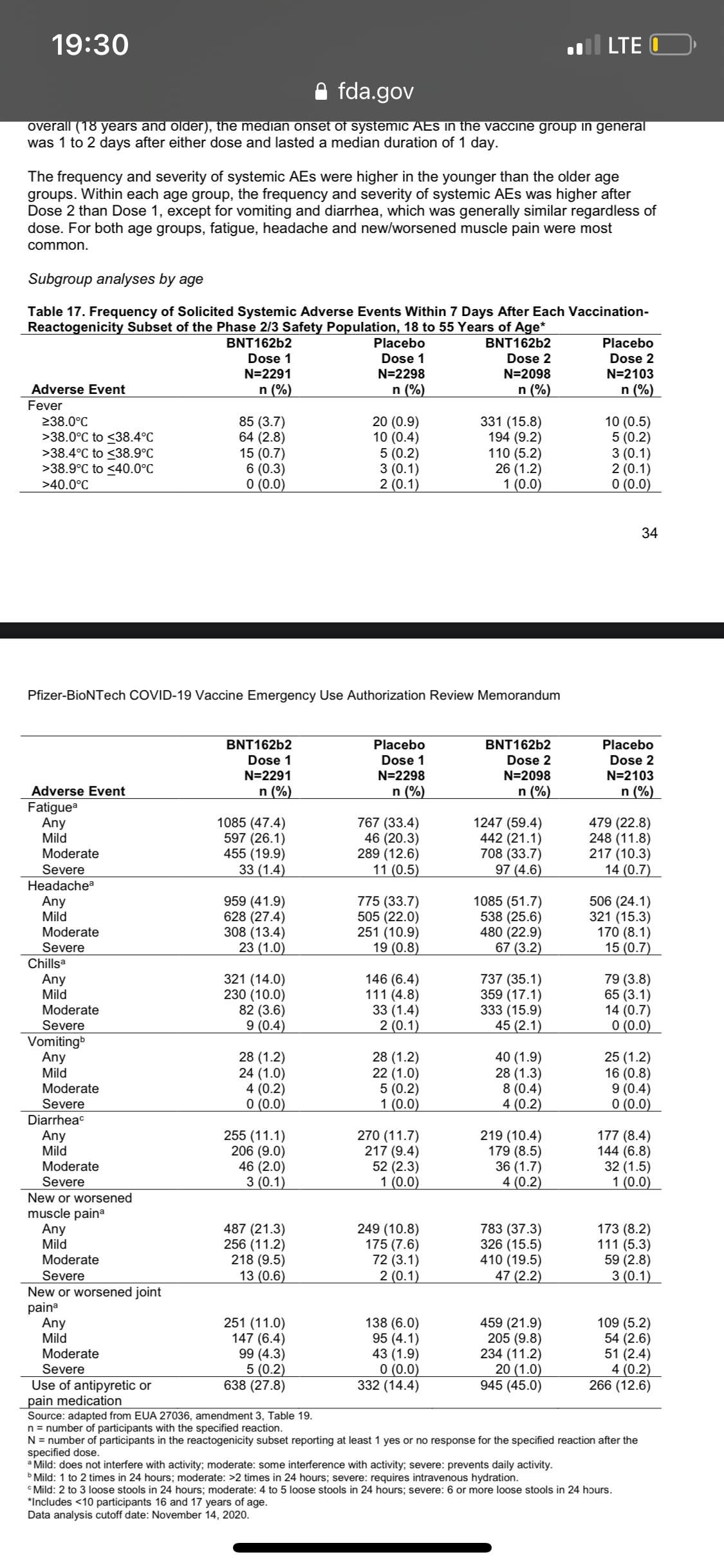
Q. Waht are the lingering effects of Covid-19?
A. Fatighue, headache, insomnia, eye and hearing changes, brain fog, hallucinations, joint pain, palpitations, syncope, loss of smell and taste, vasculitis, vomiting, colitis, cramp, etc.
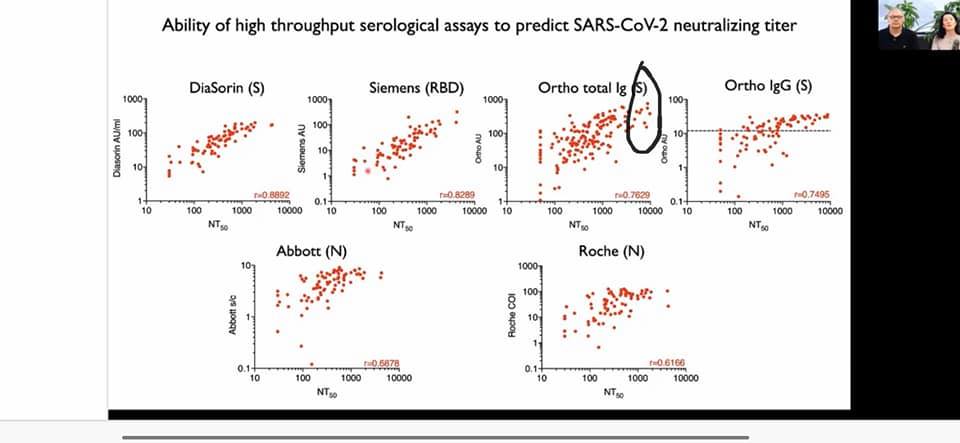
Q. Antibody testing for COVID? Is there a test for past infection and a separate test for vaccine effectiveness? Or are they the same test?
A. If you can check Spike protein antibodies then it’s due to vaccine. anti Nucleoside antibodies are due to Covid infection. you would also have anti-spike antibodies after an infection.
By day 6 post-infection, all patients showed immunoglobulin (Ig) G antibodies for the viral receptor-binding domain—a protein on the viral surface that binds to cell receptors, allowing entry and infection—and the nucleocapsid protein, the protective protein shell of the virus.
At the 100-μg dose, mRNA-1273 produced high levels of binding and neutralizing antibodies that declined slightly over time, as expected, but they remained elevated in all participants 3 months after the booster vaccination. Binding antibody responses to the spike receptor–binding domain were assessed by enzyme-linked immunosorbent assay.
The vaccines only include mRNA for the spike protein (or with coming vaccines, the spike protein). So you will not make anti-nucelocapsid antibodies post vaccination, only anti-spike. Most commercial tests are for anti-nucleocapsid, some are for both, and a few are for just anti-spike. After Covid infection, some people will make antibodies to spike and to nucleocapsid, though not all infections result in detectable antibody production. A study in the UK showed that presence of anti-spike antibodies post infection were protective against infection in health care workers.
Spike protein ab and nucleoside ab is post infection. No nucleoside ab with vaccine, just spike protein.
Pro Vaccine
Since they tested the vaccine in immunocomprimised patients with success, I figure that its worth a try to get it ahead of time instead of dealing with the long term consequences of getting covid.
The only thing that seems rushed is the red tape and funding and recruitment and disease prevalence.
I feel like we in science have gotten so used to slow progress due to less priority of funding and slower bureaucratic process. Then all of the sudden, the funding is there and the bureaucratic sluggish is minimized, so we knee-jerk jump to concerns that the science part was rushed?
Due to the public interest in COVID vaccine, COVID vaccine pushed all other vaccines back in line, funding was thrown at it like it was going out of style, the public and private sector actually cooperated in this instance. All of these factors combined for little wasted time. The mRNA technology has been years in the making.
Don't let anyone convince you the research is rushed. I wonder what the debates were like when the first polio vaccine was introduced, then the improved IPV one.
The debility and medical complications post Covid in the patients I’ve cared for are REAL. The vaccine seems much less risky than natural infection.
There is no side effect that could be as bad as getting critically ill from covid, becoming a long hauler or developing lung fibrosis in a couple of decades after getting a “mild” case of Covid-19. Data was collected on 30K people in a phase 3 well conducted robust trial. We base many medical decisions on studies conducted with a few hundred people.
Watching one of my patients die every single day of this virus. Most of them in their 50s and only minor controlled past medical histories. The unknowns of a vaccine don’t scare me. The knowns of this virus do.
They are working on developing vaccines for this class of coronavirus for nearly 2 decades. The research they were doing for the original was readily applicable to SARS-CoV2. This isn’t just some cobbled-together-at-the-last-minute treatment.
Furthermore, vaccines are by far the most thoroughly studied treatment available in modern medicine. No other medication or method of treatment is safer, nor has any other even approached the number of lives saved.
With regards to safety, prominent vaccine experts have stated that in prior vaccine trials, any serious adverse effect attributable to the vaccine has always been apparent within 6 weeks of injection.
i don’t know if the vaccine will have long term adverse effects, but I do know COVID can (e.g. cardiomyopathy, interstitial lung disease, renal impairment, and neuro cognitive decline). Therefore I’m not going to trade the known long term risks of COVID infection for the hypothetical risk of a vaccine.
we also had a good head start on this vaccine because a great deal of research has been underway for Coronaviruses since the 2002 SARS and 2012 MERS epidemics. Coronaviruses have been around since antiquity. Viruses are composed of two materials – genetic (either RNA or DNA) and proteins (usually only a few). That’s it. SARS, MERS, and COVID-19 all have a surface glycoprotein, the Spike Protein, that are very similar. This glycoprotein has been described as an easy, accessible target for natural immunity and for vaccines of all types. In other words, this didn't happen fast at all. Some of the research has almost 20 years behind it. What happened in 2020 just created a situation in which an enormous infusion of cash allowed an acceleration of work already underway.
We now have 3 emergency approved (between Europe and the USA) vaccines with the same mRNA technology and different vectors, yet all gave similar results in the same time frame with different populations of people as a randomized, double blinded, prospective trial in all of them, with the same safety profile between all three. It’s the consistency.
The vaccine provides protection [not a 100%] from getting sick with Covid and getting Covid. Even if you do get the infection it is a milder form. The mRNA is not a virus, it mimics the spike protein which In turn makes the body produce antibodies to it.
Wait for vaccine or no vaccine, no haste, vaccine hesitency
once the first round of guinea pigs get it.
It feels like a rush job with too little data and too short a time of observation on a new use of a technology.
The CDC and FDA have been stripped of any credibility at this point.
Many documented cases of people who got Covid twice, sometimes with separate strain. So it makes me wonder , if there is vaccine based on a certain strain or spike protein, it might not necessarily prevent infection from a different strain, but people may think they are protected and start engaging in risky social gatherings.
Wait 3-6 months then I can make a more educated decision of which vaccine seems the safest & most effective.
I’m going to pass on the Covid vaccine for now for myself. I’ll probably take it after 6 months or so once a lot more ppl have already gotten it and have more feedback. Don’t want to get the “first batch” of this new vaccine for a novel virus snd be a guinea pig. For next 6 months I don’t mind living in a bubble like I have for the last 9 months with social distance, mask, no travels snd 99% telemedicine.
We cannot forget the 2009 pandemic vaccine "Pandemrix" given to 30 million people caused narcolepsy in some patients (peer reviewed pubs and the book "Waking Mathilda"). I fully anticipate weird side effects in at least a small subset, possibly autoimmune. This could end up as a big siRNA experiment.
I will actively decline vaccination until version 2.0, and more data are collected on the courageous volunteers of this new technology.
Remember the rotovirus vaccine? Remember it was approved as safe? And then a year later is was withdrawn from the market for causing intusseption. Most adverse reactions from drugs and immunizations occur in the first year. I would prefer to wait a year for this reason.
4 cases of Bell's Palsy, all in vaccine group, 3 within a few days/weeks of the dose. Apparently not an unexpected rate, but interesting that placebo had 0 cases in a similar population... more data needed clearly.
It’s okay to want to see more data before injecting something in your body. It’s okay to wait and see if any other side effects.
The methods of this “survey” are about as good as the study used for the vaccine EUAs. I think we can and should be skeptical of these rapid approvals with limited data. I’ll get a vaccine at some point, but I won’t be first in line.
I’m sure the study design is compliant with industry standard.
What is lacking is 5 year safety and efficacy data.
you do know that there have been drugs put out by “major pharmaceutical companies” that have since been taken off the market right? I think people have a valid reason to be wary especially given the political and financial overtones that went into the production of the vaccine.
There’s no data supporting that this vaccination prevents or even reduces the risk of the things that people want prevented to get back to normal: hospitalization and transmission. So essentially with current info, you’d be getting vaccinated to avoid headache, runny nose, cough, fever (their endpoints in the study)
Would like to see more data ruling out vaccine enhanced disease. Prelim animal studies are reassuring but no good human data.
only 8 people in the Pfizer trial got the vaccine and got COVID. While that’s good in the sense that getting COVID was prevented, there’s very little data on what happens if you get COVID after the vaccine. Vaccine enhanced disease (worsened disease if it naturally occurs after the vaccine) had been a huge barrier in development of vaccine for other respiratory pathogens like RSV and SARS.
I’d like to see a bit more data on this aspect in a bigger population so I hope this population is still being studied and followed.
https://buildvaccinetrust.wordpress.com/Information/
There is no hard human data showing decreased in hospitalization or transmission. However I think you need to extrapolate. Moderna enrolled 30,000 patients, half getting the vaccine. 5 infections happened on the vaccinated group and 90 happened in the placebo group. Out of those 90 infections 11 were considered "severe"; while none of the 5 patients in the vaccine group had "severe disease". Of course, I would love to know precisely how they defined severe. Pfizer's vaccine, out of 43,000 subjects, 170 contracted the disease with only 8 subjects on the vaccine group. So clearly, just because they reduce the risk of symptomatic COVID you can expect a decrease in hospitalization rate. Do patients who are vaccinated AND get the illness are at the same/lower/higher risk of having illness requiring hospitalization? Moderna data suggests they have lower risk, but numbers of people with illness are low. On the transmission front, none of these studies were designed to show a drop in transmission. You can imagine how difficult this would be to orchestrate. Still, in mice, the Moderna vaccine had sterilizing immunity. In primates, it decreased the risk of infection after challenge and the animals that got infected had no symptoms and shed virus for decreased period of time.
Nobody has concerns about where the funding came from for the Pfizer vaccine?
The vaccine has not been adequately vetted and tested and to tell people that we should be just taking it to set an example is not scientific or even common sense.
Has only been tested for a reduction of severity of symptoms from day 7 to 8 weeks. They should and need to test post vaccinated individuals for viral PCR to actually see if it has any potential to prevent actually acquiring the virus. Otherwise all you’re potentially doing is increasing the likelihood being an asymptomatic carrier if infected. I find it odd that they are not testing for effectiveness of this vaccine by following these individuals and routinely testing them for Covid to see if it actually prevents acquiring the infection in the first place at all. They need to include this data to provide useful information and recommendations for who should be vaccinated and who should not. Definitely don’t think anyone should be judging physicians or other individuals who are opting to not receive the vaccine currently.
They did not routinely test for antibodies or for COVID PCR positivity post vaccine in the studies. They only waited if someone reported they had Covid symptoms and then they would test them for active disease. The studies truly do not come anywhere close to proving or implying that the vaccines would actually prevent infection on any level. They should’ve performed antibody testing and routine PCR testing on the study subjects to see if the vaccines were able to reduce infection rate at all, but they did not. As physicians we should demand more data and continued following of patients that were in these studies, and the newly vaccinated first line candidates as well. Hopefully at least we will see what the trends are from other physicians in groups such as this what they are seeing clinically and possibly testing post vaccinated patients periodically via PCR for active infections as well as for antibodies to see if they mount an immune response and for how long.
I think it’s easy castigate people who have questions or concerns. Even the officials promoting vaccination have unanswered questions. I truly do hope that these are the miracle vaccines we all want them to be. However, there are countries who have controlled this pandemic much more effectively and had life this summer. Some of them still have life. Others are locking down again for numbers that we could only dream of having in this country. Many people in this country continue to live as if it doesn’t exist even as we approach 500k deaths in the first quarter of the year with no way statistically of stopping that at this point. These vaccines are a slow-moving lifeboat at best, when relatively short-term compliance with public health measures would have been a flight back to normalcy.
Not being able to sue vaccine makers for adverse reactions is not reassuring, despite mainstream media echoing how safe it is.
Mask is working for me until now. I will keep my mask and social distancing.
https://www.youtube.com/watch?v=KMc3vL_MIeo&list=WL&index=2
Dr. Ramsis Ghaly FB post:
Antibody Testing Prior and After Vaccination Is Not Being Mandated or Part of the Requirements! Why???
Written by Ramsis Ghaly
It is already well established mandatory guidelines, except for unknown reasons in COVID vaccination, if someone receiving hepatitis vaccine or tuberculosis TB, to be tested prior vaccination to see if he or she ever got infected before and has antibodies already. And also must be tested after vaccination to confirm the effectiveness of vaccine and development of the antibodies in that particular subject!
It is the right thing to do. It is patient safety. It is for better industry pharmaceutical oversight control. It is part of keeping them honest! It is for accurate data collection! It won’t delay the process at all! So why isn’t done or implemented ASAP! After all you are authorizing use of vaccine to the entire world that is not even completely studied, might as well follow the quantitative accurate results for free to audit and to ensure what are you giving the public that the vaccine is safe and is doing what suppose to do!!
For COVID vaccine prior and after, obligatory testing has been waived and been told we must assume that 95% effectiveness! A company study results does not mean the public is indeed harmonious group with the previous pharmaceutical operated study!! In addition, certainly recipients would like to know if vaccination is really needed especially if already antibodies in the system from previous sub clinical infection! And also receipents would like to know if the vaccine was effective or he or she is of the 5% non responders! Why the recipient must pay to be tested if it should be part of the CDC mandation on experimental vaccine conducted by the pharmaceutical?
Isn’t considered violation of patients Safety in support of Industry Regulation Rules!
So far volunteers are receiving vaccine without being tested before and after! And if any of them interested, they must pay for it! In the same time no steps have been taken to examine accuracy of the vaccine to the recipients!
As the experimental vaccination continues, the pharmaceuticals should schedule the volunteers for free antibody Titre test prior and after vaccination! And they must keep the books straight and clean? CDC and public safety shouldn’t allow short cuts when it comes to patients safety facing industry involvement!
Otherwise how you test the effeviness of the experimental vaccine you are giving to the public in an emergency basis? How are you studying the vaccine? What data you are collecting to keep the records straight and examine the results??? Are we giving the pharmaceuticals free path here and taking advantage of the imposed public emotional vulnerability fear and terrors!
Nowadays, people are getting vaccinated rushing without being tested prior to see if they already had COVID antibodies and therefore vaccination is needed to start with or after to see if the vaccine was effective! They decided if isn’t required and it us individual preference and must pay for it (average $500) Each vaccine recipient must also be tested after vaccination to confirm effectiveness of vaccine and development of adequate antibodies
Ideally each one should demand to be tested for free prior to receiving the vaccine and after receiving the vaccine for antibody title as all other historic vaccine guidelines!
American Society of Anesthesiologists and Anesthesia Patient Safety Foundation Joint Statement on Elective Surgery and Anesthesia for Patients after COVID-19 Infection December 8, 2020
American Society of Anesthesiologists (ASA)
Anesthesia Patient Safety Foundation (APSF) Since hospitals are able to continue to perform elective surgeries while the COVID-19 pandemic continues, determining the optimal timing of procedures for patients who have recovered from COVID-19 infection and the appropriate level of preoperative evaluation are challenging given the current lack of evidence or precedent. The following guidance is intended to aid hospitals, surgeons, anesthesiologists, and proceduralists in evaluating and scheduling these patients. It is subject to change as new evidence emerges.
In general, all non-urgent procedures should be delayed until the patient has met criteria for discontinuing isolation and COVID-19 transmission precautions and has entered the recovery phase. Elective surgeries should be performed for patients who have recovered from COVID-19 infection only when the anesthesiologist and surgeon or proceduralist agree jointly to proceed. What determines when a patient confirmed to have COVID-19 is no longer infectious?
The Centers for Disease Control and Prevention (CDC) provides guidance for physicians to decide when transmission-based precautions (e.g., isolation, use of personal protective equipment and engineering controls) may be discontinued for hospitalized patients or home isolation may be discontinued for outpatients.
Patients infected with SARS-CoV-2, as confirmed by reverse transcriptase-polymerase chain reaction (RT-PCR) testing of respiratory secretions, may be asymptomatic or symptomatic. Symptomatic patients may be further sub-classified into two groups depending upon symptom severity. Table 1 provides definitions of these COVID-related illness levels of severity.
Patients with mild to moderate symptoms* (generally those without viral pneumonia or oxygen saturation below 94 percent)
Patients who experienced severe or critical illness** due to COVID-19 (e.g., pneumonia, hypoxemic respiratory failure, septic shock).Severely immunocompromised patients***, whether suffering from asymptomatic or symptomatic COVID-19, are considered separately.
Current data indicate that, in patients with mild to moderate COVID-19, repeat RT-PCR testing may detect SARS-CoV-2 RNA for a prolonged period after symptoms first appear. However, in these patients, replication-competent virus has not been recovered after 10 days have elapsed following symptom onset. Considering this information, the CDC recommends that physicians use a time- and symptom-based strategy to decide when patients with COVID-19 are no longer infectious.
For patients with confirmed COVID-19 infection who are not severely immunocompromised and experience mild to moderate symptoms*, the CDC recommends discontinuing isolation and other transmission-based precautions when:
At least 10 days have passed since symptoms first appeared.
At least 24 hours have passed since last fever without the use of fever-reducing medications.
Symptoms (e.g., cough, shortness of breath) have improved.For patients who are not severely immunocompromised and have been asymptomatic throughout their infection, isolation and other transmission-based precautions may be discontinued when at least 10 days have passed since the date of their first positive viral diagnostic test.
In approximately 95 percent of severely or critically ill patients (including some with severe immunocompromise), replication-competent virus was not present after 15 days following the onset of symptoms. Replication-competent virus was not detected in any severely or critically ill patient beyond 20 days after symptom onset.
Therefore, in patients with severe to critical illness** or who are severely immunocompromised***, the CDC recommends discontinuing isolation and other transmission-based precautions when:
At least 10 days and up to 20 days have passed since symptoms first appeared.
At least 24 hours have passed since the last fever without the use of fever-reducing medications.
Symptoms (e.g., cough, shortness of breath) have improved.Consultation with infection control experts is strongly advised prior to discontinuing precautions for this group of patients. Clinical judgment ultimately prevails when deciding whether a patient remains infectious. Maintaining transmission-based precautions and repeat RT-PCR testing may be appropriate if clinical suspicion of ongoing infection exists. The utility of repeat RT-PCR testing after improvement in symptoms is unknown as patients will frequently remain at least intermittently positive for weeks to months.
If a patient suspected of having SARS-CoV-2 infection is never tested, the decision to discontinue transmission-based precautions can be made using the symptom-based strategy described above.
Other factors, such as advanced age, diabetes mellitus, or end-stage renal disease, may pose a much lower degree of immunocompromise; their effect upon the duration of infectivity for a given patient is not known.
Ultimately, the degree of immunocompromise for the patient is determined by the treating provider, and preventive actions are tailored to each individual and situation. What is the appropriate length of time between recovery from COVID-19 and surgery with respect to minimizing postoperative complications?
The preoperative evaluation of a surgical patient who is recovering from COVID-19 involves optimization of the patient’s medical conditions and physiologic status. Since COVID-19 can impact virtually all major organ systems, the timing of surgery after a COVID-19 diagnosis is important when considering the risk of postoperative complications.
There are limited data now that address timing of surgery after COVID-19 infection. One study found a significantly higher risk of pulmonary complications within the first four weeks after diagnosis (1). An upper respiratory infection within the month preceding surgery has previously been found to be an independent risk factor for postoperative pulmonary complications (2). Patients with diabetes are more likely to have severe COVID-19 disease and are more likely to be hospitalized (3,4). Studies conducted during the 2009 influenza A H1N1 pandemic found that pulmonary function continues to recover up to three months after ARDS (5).
Given this current knowledge base, wait times before surgery can be reasonably extrapolated and are a suggested starting point in the preoperative evaluation of the COVID-19-recovered patient.
The timing of elective surgery after recovery from COVID-19 utilizes both symptom- and severity-based categories. Suggested wait times from the date of COVID-19 diagnosis to surgery are as follows:
Four weeks for an asymptomatic patient or recovery from only mild, non-respiratory symptoms.
Six weeks for a symptomatic patient (e.g., cough, dyspnea) who did not require hospitalization.
Eight to 10 weeks for a symptomatic patient who is diabetic, immunocompromised, or hospitalized.
Twelve weeks for a patient who was admitted to an intensive care unit due to COVID-19 infection.These timelines should not be considered definitive; each patient’s preoperative risk assessment should be individualized, factoring in surgical intensity, patient co-morbidities, and the benefit/risk ratio of further delaying surgery.
Residual symptoms such as fatigue, shortness of breath, and chest pain are common in patients who have had COVID-19 (6,7). These symptoms can be present more than 60 days after diagnosis (7). In addition, COVID-19 may have long term deleterious effects on myocardial anatomy and function (8). A more thorough preoperative evaluation, scheduled further in advance of surgery with special attention given to the cardiopulmonary systems, should be considered in patients who have recovered from COVID-19 and especially those with residual symptoms. Is repeat SARS-CoV-2 testing needed?
At present, the CDC does not recommend re-testing for COVID-19 within 90 days of symptom onset (9). Repeat PCR testing in asymptomatic patients is strongly discouraged since persistent or recurrent positive PCR tests are common after recovery. However, if a patient presents within 90 days and has recurrence of symptoms, re-testing and consultation with an infectious disease expert can be considered.
Once the 90-day recovery period has ended, the patient should undergo one pre-operative nasopharyngeal PCR test ideally ≤ three days prior to the procedure. References
COVIDSurg Collaborative. Delaying surgery for patients with a previous SARS‐CoV‐2 infection. BJS 2020; 107: e601–e602. https://doi.org/10.1002/bjs.12050
Canet J, Gallart L, Gomar C, et al. Prediction of postoperative pulmonary complications in a population-based surgical cohort. Anesthesiology 2010;113:1338. https://doi.org/10.1097/ALN.0b013e3181fc6e0a
Guan WJ, Liang WH, Zhao Y, et al. Comorbidity and its impact on 1590 patients with Covid-19 in China: a nationwide analysis. Eur Respir J 2020. DOI: 10.1183/13993003.00547-2020
Petrilli CM, Jones SA, Yang J, et al. Factors associated with hospital admission and critical illness among 5279 people with coronavirus disease 2019 in New York City: prospective cohort study. BMJ 2020;369:m1966 doi: https://doi.org/10.1136/bmj.m1966.
Hsieh M-J, Lee W-C, Cho H-Y, et al. Recovery of pulmonary functions, exercise capacity, and quality of life after pulmonary rehabilitation in survivors of ARDS due to severe influenza A (H1N1) pneumonitis. Influenza and other respiratory viruses. Apr 2018. https://doi.org/10.1111/irv.12566
Tenforde MW, Kim SS, Lindsell CJ., et al. Symptom Duration and Risk Factors for Delayed Return to Usual Health Among Outpatients with COVID-19 in a Multistate Health Care Systems Network – United States, March-June 2020. MMWR 2020 Jul 31;69(30):993-998. https://dx.doi.org/10.15585%2Fmmwr.mm6930e1
Carfi A, Bernabei R, Landi F., et al. Persistent Symptoms in Patients After Acute COVID-19. JAMA July 9, 2020. doi:10.1001/jama.2020.12603
Puntmann VO, Carerj ML, Wieters I, et al. Outcomes of Cardiovascular Magnetic Resonance Imaging in Patients Recently Recovered From Coronavirus Disease 2019 (COVID-19). JAMA Cardiol. 2020;5(11):1265-1273. doi:10.1001/jamacardio.2020.3557
https://www.cdc.gov/coronavirus/2019-ncov/hcp/duration-isolation.html Accessed Oct 28, 2020Table 1: Definitions for Severity Levels of COVID-Related Illness
The studies used to inform the guidance in this joint statement do not clearly define “severe” or “critical” illness. The definitions in the National Institutes of Health (NIH) COVID-19 Treatment Guidelines (cited under references below) are suggested to categorize disease. The highest level of illness severity experienced by the patient at any point in their clinical course should be used.
-
Mild Illness: Signs and symptoms of COVID-19 (e.g., fever, cough, sore throat, malaise, headache, muscle pain) without shortness of breath, dyspnea, or abnormal chest imaging.
-
Moderate Illness: Evidence of lower respiratory disease by clinical assessment or imaging and oxygen saturation (SpO2) ≥94 percent on room air at sea level.
** Severe Illness: Respiratory rate >30 breaths per minute, SpO2 <94 percent on room air at sea level (or, for patients with chronic hypoxemia, a decrease from baseline of >3 percent), a ratio of arterial partial pressure of oxygen to fractional inspired oxygen (PaO2/FiO2) <300 mmHg, or lung infiltrates involving >50 percent of the lung fields.
** Critical Illness: The presence of respiratory failure, septic shock, and/or multiple organ dysfunction.
*** The studies used to inform this guidance did not clearly define “severely immunocompromised.” For the purposes of this guidance, “severely immunocompromised” refers to patients:
Currently undergoing chemotherapy for cancer.
Within 1 year of receiving a hematopoietic stem cell or solid organ transplant.
Having untreated HIV with a CD4 T lymphocyte count <200.
Having a combined primary immunodeficiency disorder.
Treated with prednisone >20mg/day for more than 14 days.Reference sources from CDC and NIH websites as of 22 Sept 2020: Interim Clinical Guidance for Management of Patients with Confirmed Coronavirus Disease (COVID-19) https://www.cdc.gov/coronavirus/2019-ncov/hcp/clinical-guidance-management-patients.html
Overview of testing https://www.cdc.gov/coronavirus/2019-ncov/hcp/testing-overview.html
Discontinuation of Transmission-Based Precautions and Disposition of Patients with COVID-19 in Healthcare Settings (Interim Guidance) https://www.cdc.gov/coronavirus/2019-ncov/hcp/disposition-hospitalized-patients.html
Duration of Isolation and Precautions for Adults with COVID-19 https://www.cdc.gov/coronavirus/2019-ncov/hcp/duration-isolation.html?CDC_AA_refVal=https%3A%2F%2Fwww.cdc.gov%2Fcoronavirus%2F2019-ncov%2Fcommunity%2Fstrategy-discontinue-isolation.html
National Institutes of Health (NIH) COVID-19 Treatment Guidelines https://www.covid19treatmentguidelines.nih.gov/whats-new/
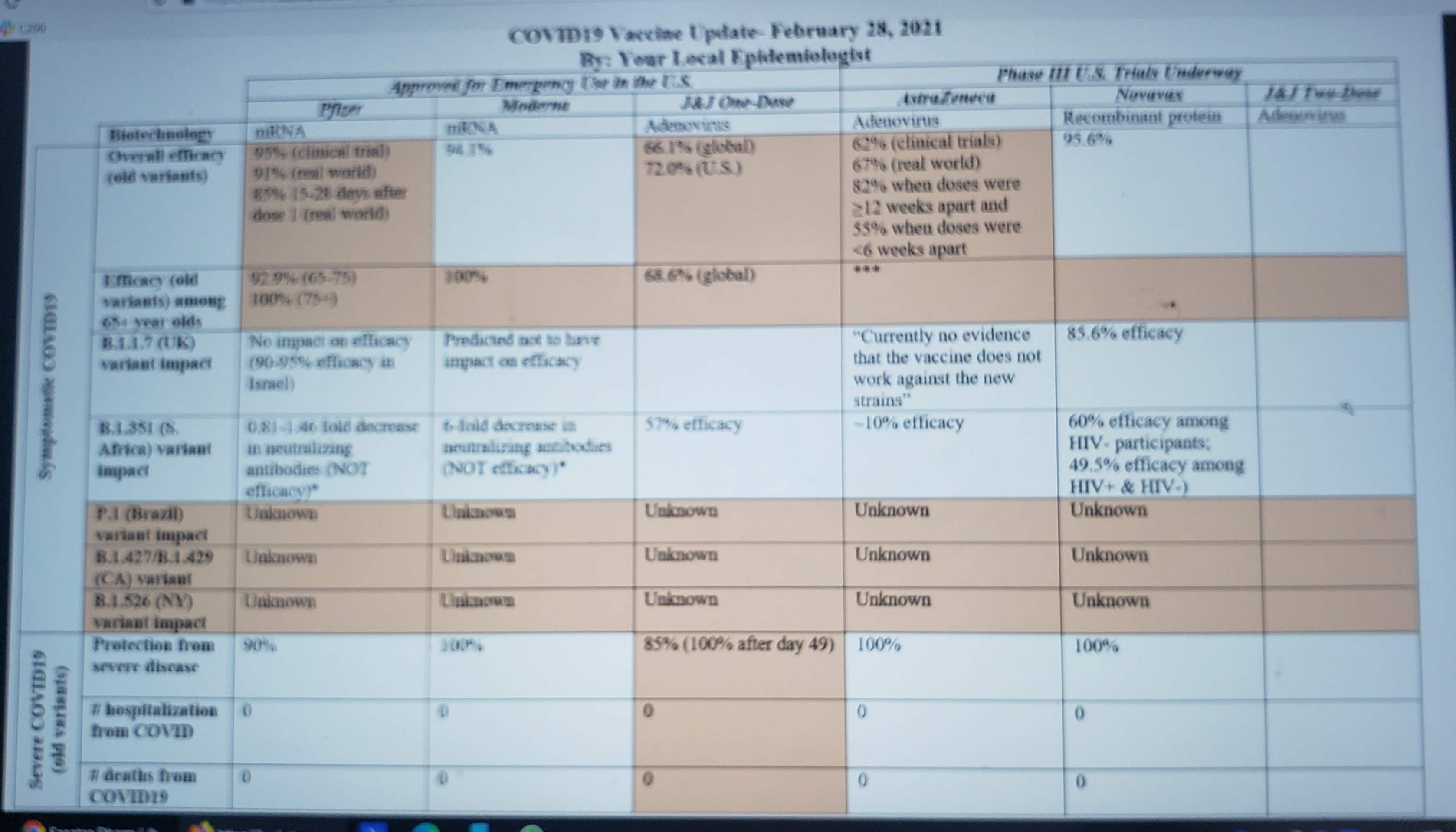
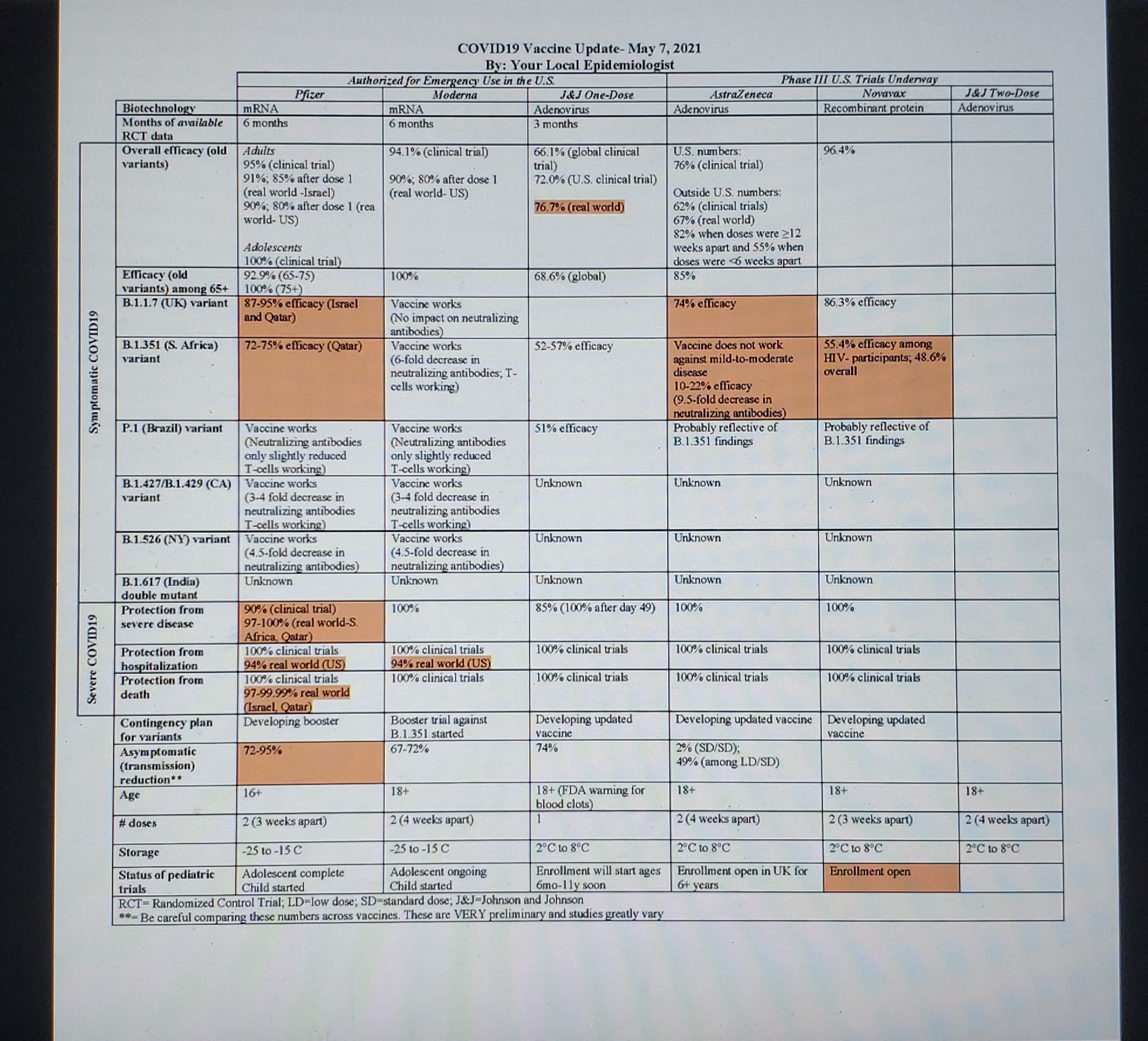
Covid vaccine in India
Whatsapp forwards:
FAQ’s on Covid Vaccines:
-
When is the Corona vaccine likely to be available? Probably the Government will get it by January and the private market by March.
-
Do we all need to take it? Yes, all should take it.
-
Who will get it first? It will be prioritised. First frontline workers and first responders like paramedical staff, civil servants, police, army, politicians and their relatives will get it first. People more than 50 years of age and those with co-morbidities like diabetes, HT, transplant and chemotherapy patients will get it next. Then will be healthy adults, teenagers, children and last neonates if at all.
-
How will it be given? Through public and private centres, by doctors, dentists, nurses and trained paramedics.
-
What is the recommended dose and schedule? Two doses given 21 days or 28 days apart depending on vaccine used.
-
What if I take only one dose? One dose will give you only partial protection of maybe 60-80% and will not last long enough. For complete protection you must take two doses at recommended intervals.
-
What if I forget to take the second dose? Should I take the first again? Just take the second dose at the earliest. No need to repeat the first dose.
-
Are both doses same? In most of the vaccines it will be the same dose given twice. However, Sputnik- V vaccine has both doses as different vector viruses, so will be marked as dose 1 and 2. Oxford-AZ vaccine may also come out with first dose as half dose.
-
Do you need to take it even if you had Corona? After how many days of getting cured?
Yes. But that will be last in the priority list. You can let others take who probably need more than you. You might need it earlier if you did not develop an antibody response.
-
Can it be administered to an individual who has received plasma as treatment for Covid? The donor plasma contains anti Covid-19 antibodies and may suppress the immune response to the vaccine. As it is, those who have recovered from Covid-19 may not need the vaccine in the early phases.
-
Can a pregnant lady or a lactating mother take the vaccine? No company has yet tested the vaccine in pregnancy. CDC has advised against giving the vaccine to pregnant and lactating mothers. UK authorities have advised women not to get pregnant for two months after the shot. Since the vaccines available till now are not live vaccines, it should not cause any problem if given inadvertently.
-
Can a diabetic patient take the vaccine? Yes, in fact diabetes has been established as a risk factor for severe disease and all diabetic patients must get vaccinated on priority.
-
If offered a choice of vaccines, which one should I take? All vaccines are offering equal efficacy although local reactions may be different. Take whatever available. Think positive that at least you are being offered a vaccine ahead of others. Indian manufactured vaccines will be more suitable for our population as they are cheaper and can be kept at 2-8 degree Celsius. The mRNA vaccines require a storing temp of -70 (Pfizer) and -20 (Moderna) which may be difficult to maintain in summer months.
-
How many days after getting vaccine, would I develop protection? Best protection starts 10 days after second dose. Efficacy is around 70-90% against all severity and 100% against hospitalization. Immediate aim is to prevent hospitalisation and mortality.
-
How long will the vaccine provide immunity? It is a new virus, new technology vaccine, so we don't know. After follow-ups of these vaccinated population and their antibodies for a couple of years, we would be wiser. The need for boosters and when will they be required, will be decided after these follow ups and mathematical modelling.
-
Children of what age can be vaccinated? Is the dose same as adults or lesser dose to be given? Trials done till now have been for adults above 18 only. Now trials for children above 12 have started. Doses will be decided only after trials are done on younger children and infants.
-
Can it be given to immunocompromised individuals? The mRNA vaccine and inactivated vaccines are safe. AZ and Sputnik-V adenovirus vector vaccines are also safe as they are nonreplicating viral vector vaccines. Live vaccines and replicating viral vector vaccines will have to be avoided.
-
What are the side effects expected? The side effects reported by the trial population are mostly mild Covid like symptoms like some fever and fatigue. Local injection site pain and induration is also reported. Reports of transverse myelitis and facial palsy have not been found to be related to the vaccine. Generally, all vaccines are safe. Although these vaccines have been made in record time, the testing methodology and procedures have not been compromised.
-
I am allergic to egg. Can I take the vaccine? Egg cell lines are not used for production of these vaccines. They can be taken safely even if you are allergic to egg.
-
I heard that it has pig or monkey products? I am a pure Vegetarian. The new vaccines manufactured these days are devoid of any such products.
-
In the past vaccines have been linked to Autism. What about these? In 1985 there was a paper linking MMR with autism. Millions of children followed up after that have conclusively proven that there is no relationship between vaccines and autism. All vaccines are extremely safe with minimal temporary side effects.
-
There are messages going around that mRNA from vaccine gets incorporated into the human genome and alter our genetic structure. Is that true? mRNA vaccine carries a message to the cell to produce spike protein which induces antibody production. It does what it is directed to do. Till date there have been no adverse events reported.
-
What is the interaction of alcohol and Covid vaccine? Excessive alcohol can reduce the immune responses to vaccines. Since Russians are known for heavy drinking, their government has advised to avoid drinking two weeks prior to first dose and 6 weeks after the second dose. The Sputnik vaccine is given as two doses 21 days apart. Occasional glass of wine or beer will not interfere with the immune response.
-
Soon the virus will mutate and we will need another vaccine. Should we not wait? Till now the virus has not shown tendency to mutate like the Flu virus. Moreover, the vaccines being developed have taken this into consideration and should still work.
-
What if I do not want to take the vaccine? Will it be made mandatory? In majority of countries, it will not be mandatory. You have to choose between the new viral disease with no specific treatment and a new vaccine. Choice is yours. As initially there will be a huge demand supply gap, by not taking a vaccine you can help others.
-
If I fall in the category of priority list by being a senior citizen or with a co-morbid condition, how do I contact the appropriate vaccination authority? Soon there will be a website and an app ‘CoWIN’ where you will be able to register with your relevant details.
-
_What is CoWIN? It is the world’s first, digital, end to end, vaccine distribution and management system. It includes beneficiary registration, authentication, document verification, session allocation, AEFI reporting and certificate generation. Once the vaccine is available, it will generate a SMS informing the beneficiary. The vaccine centre itself will be managed by five people and will give maximum 100 vaccines per day. The vaccine recipient has to wait for 30 minutes before leaving the centre post-vaccination.
-
What are the different types of Corona vaccines likely to be available for use in near future? Covishield , by Serum Institute of India (Oxford AstraZeneca) is a non- replicating viral vector vaccine. These are viruses that have been modified to act as delivery systems that carry the viral antigens to our immune cells. Chimpanzee adenovirus is the vector used to deliver the corona virus antigen in the SII vaccine and human adenoviruses in Sputnik V (Russian vaccine, made in India by Dr Reddy’s lab). Covaxin , by Bharat Biotech India Ltd is a whole cell inactivated vaccine. Most of the current vaccines being used in Pediatric immunization, are made by this technology. Since these are killed viruses, they produce immunity, but cannot cause the disease. Pfizer and Moderna vaccines from USA, consist of messenger RNA molecules. The carry the code message which induces the human cell to manufacture spike protein of the Corona virus. These proteins are recognised by our immune system to produce antibodies. Other Indian companies like Biological E, Cadila Healthcare and Genova are also in advanced stage of vaccine development.
-
Can I roam around without a mask once I am vaccinated? No, not as of now. One may do so only when the majority of the population has either got the disease or received the vaccine. This means the population has developed herd immunity.
-
Are newer and better Covid vaccines expected in near future? As of December 2020, more than 250 vaccines are under trial in different phases. A lot of research is underway to develop newer delivery methods also. Nasal spray vaccine is probably the most promising. A multi dose nasal spray delivery device can be very convenient and economical. It will produce local IgA antibodies and block the virus at entry itself. It will reduce nasal colonisation and thus prevent transmission of disease also. Unfortunately, since it will be a live vaccine, it will need maximum and most stringent trials and thus will take longest time to hit the market. Covid-19 is still a new disease and we are still learning. The facts mentioned above are as of 14 December 2020. Please re-check the facts before taking a Covid vaccine shot.
No vaccine gives 100% protection. Also, a vaccinated person may not develop disease but may transmit it to others. Please continue to wear mask, observe physical distance and sanitize hands for some more time.
Important points from IAP Webnar on Covid Vaccination.
Speakers: Dr T Jacob John, Dr Naveen Thacker, Dr Nitin, Dr Vipin Vashishtha
1.Vaccine is better than no vaccine.
2.Take vaccine as scheduled in trials.
3.Side effects are minimal with both the Indian vaccines
4.Herd immunity will come eventually but cases will remain though in very small numbers.
5.UK strain is less likely to create another wave in India.
6.After September 2020, when 30% attained antibodies, the peak of incidence per day started decreasing.
7.This covid situation will last for few years.
8.Kids 2 years and above should also be vaccinated with Covaxin, this will reduce school to kids to parents spread. This recommendation has been made by eminent Pediatiricans. GOI has yet to accept this recommendation.
9.The claim that Covaxin will more suitable against mutants is not proven.It will be clear once complete data is available.
10.Oxford vaccine data is not very clear cut and clean.It has been done in many countries and data is difficult to reconcile/ correlate.
11.UK has started using Oxford Vaccine, so we should have more data in near future.
12.Covaxin has not shown phase 3 trial reports,still it should be tried as GOI must have gone through the details and must have found it worth.
13.More than 50% Covaxin voulenteers had no side effect.so it means 1200 people had no side effect at all.
14.Two doses should be taken and not one as done in trials.
15.Any vaccine if taken even one dose, will prevent serious covid after two weeks. Which means partial immunity is better than no immunity.
16.Side effect of both the vaccine is like fever and pain in few recipients.Its minimal so far.
17 Even 50-60% effective vaccine is very useful.
18.Even after 90% people have herd immunity by vaccine or disease per se, disease will still remain though incidence will be minimal.
19.Things will be more clear once both the vaccines are used for 1-2 months. So Phase 1 where all Healthcare providers will be vaccinated will give us much better understanding.
- Universal immunisation and commercial availability of Vaccine (on demand) is still months/ years away.
Lastly, no one will be vaccinated forcefully. Every beneficiary has the right to accept or refuse the vaccine.
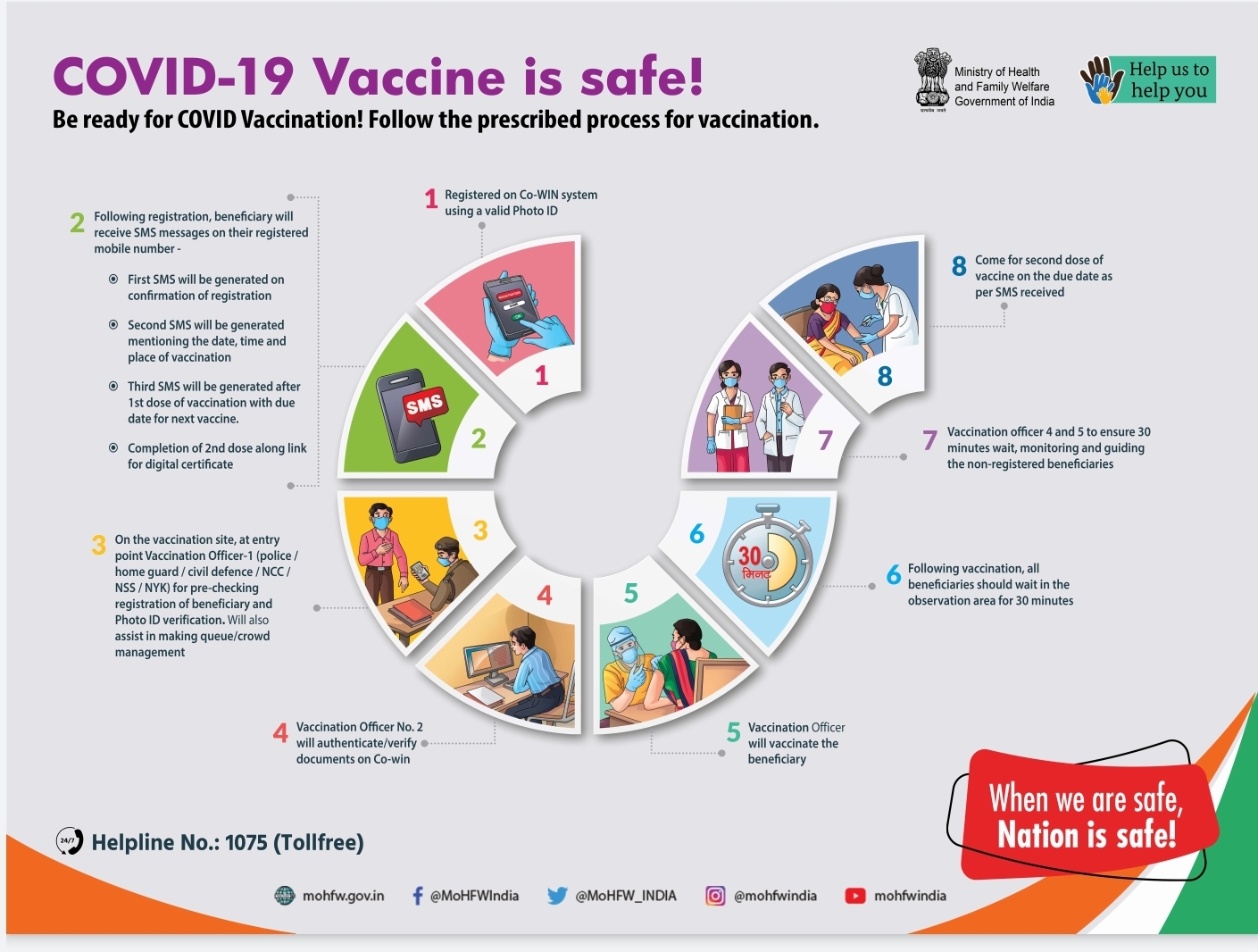
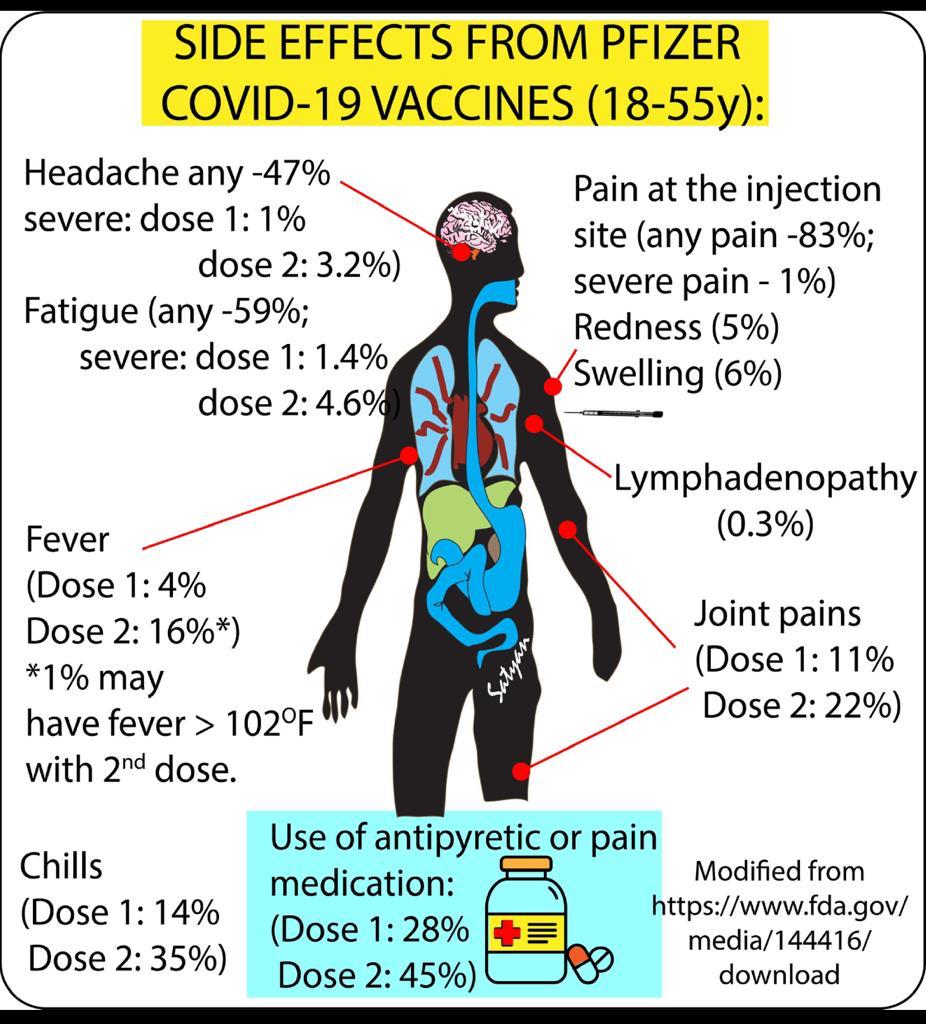
🏼 ABHIYAN 🏼
एक प्रयास...
भारत सरकार के स्वास्थ्य मंत्रालय की ओर से जारी कोविड टीकाकरण पर प्रश्नोत्तर
✍🏽सवाल 1- कोविड-19 टीके की कितनी डोज होगी और इसे कितने अंतराल में लेना पड़ेगा?
जवाब: हर व्यक्ति को टीके की दो डोज लेनी पड़ेगी जो 28 दिन के अंतराल पर दी जायेगी।
✍🏽सवाल 2- टीका लेने के बाद ऐंटीबॉडी कबतक विकसित हो जायेगी?
जवाब: आमतौर पर कोविड- 19 टीके की दूसरी डोज लेने के दो सप्ताह बाद एंटीबॉडी विकसित हो जाती है।
✍🏽सवाल 3- टीका लेने के लिए पंजीकरण कराने के वास्ते पात्र लाभार्थियों को कौन से दस्तावेज देने होंगे।
जवाब: पंजीकरण के लिए लाभार्थी निम्नलिखित फोटो आई डी वाला कोई भी एक दस्तावेज दे सकते हैं। जैसे👇
a- ड्राइविंग लाइसेंस/मतदाता पहचान पत्र/पैन कार्ड/पासपोर्ट/रोजगार कार्ड/पेंशन दस्तावेज।
b- श्रम मंत्रालय की योजना के तहत जारी स्वास्थ्य बीमा स्मार्ट कार्ड
c- मनरेगा कार्ड
d- सांसदों और विधायकों को जारी सरकारी पहचान पत्र
e- बैंक/डाकघर से जारी पास बुक
f- केंद्र/राज्य सरकार और पब्लिक लिमिटेड कम्पनियों की ओर से जारी पहचान पत्र।
✍🏽सवाल 4- क्या कोई व्यक्ति बिना पंजीकरण कराए टीका ले सकता है?
जवाब: नहीं। कोविड-19 वैक्सीन लेने के लिए पंजीकरण अनिवार्य है। पंजीकरण कराने के बाद ही किस दिन, कहां और किस समय पर टीका लगाया जाएगा इसकी जानकारी साझा की जाएगी।
✍🏽सवाल 5- टीका लगने से पहले यदि कोई व्यक्ति पहचान पत्र प्रस्तुत नहीं कर पाता है तो क्या उस स्थिति में उसे टीका लगाया जा सकता है?
जवाब: टीका लगाए जाने से पहले पंजीकरण के लिए पहचान पत्र प्रस्तुत करना जरूरी है ताकि यह सुनिश्चित किया जा सके कि सही लाभार्थी को टीका लगाया जा रहा है।
✍🏽सवाल 6- लाभार्थी को टीकाकरण के दिन की जानकारी कैसे मिलेगी?
जवाब: ऑनलाइन पंजीकरण के बाद, लाभार्थी के मोबाइल नंबर पर SMS भेजकर टीकाकरण के दिन, समय और स्थान की जानकारी दी जाएगी।
✍🏽सवाल 7- टीकाकरण पूरा होने के बाद भी क्या लाभार्थी को उसके टीकाकरण से संबंधित ताजा जानकारी मिलती रहेगी?
जवाब: हां। कोविड टीकाकरण का चक्र पूरा होने के बाद लाभार्थी को उसके मोबाइल नंबर पर SMS भेजा जाएगा। मोबाइल नम्बर पर टीकाकरण पूरा होने का QR कोड आधारित एक प्रमाण-पत्र भी जारी किया जाएगा।
✍🏽सवाल: 8- कोई व्यक्ति कोविड का टीका कहां लगवा सकता है।
जवाब: सरकार द्वारा निर्धारित टीकाकरण केंद्र पर जाकर।
✍🏽सवाल: 9- कोविड के टीके को एक जगह से दूसरी जगह ले जाने, रखने और लगाने की क्या व्यवस्था की गई है।
जवाब: इस पूरी प्रक्रिया के लिए कोल्ड चेन का पालन किया जाएगा।
✍🏽सवाल: 10- अगर कोई व्यक्ति कैंसर, मधुमेह और उच्च रक्तचाप जैसी बीमारियों के लिए दवा ले रहा है तो क्या वह कोविड का टीका लगवा सकता है?
जवाब: हां। ऐसी एक या उससे अधिक बीमारियों वाले व्यक्ति को कोविड का सबसे ज्यादा खतरा हो सकता है। ऐसे में उसे कोविड का टीका जरूर लगवाना चाहिए।
✍🏽सवाल: 11- क्या स्वास्थ्य कर्मियों/अग्रिम पंक्ति के कार्यकर्ताओं के परिवार वालों को भी टीका लगाया जाएगा।
जवाब: शुरूआती स्तर पर टीके की सीमित आपूर्ति को ध्यान में रखते हुए पहले प्राथमिकता वाले लोगों को टीका लगाया जाएगा और बाद में चरणबद्ध तरीके से जरूरतमंद लोगों को यह उपलब्ध कराया जाएगा।
✍🏽सवाल 12- क्या सबको एक साथ कोविड का टीका लगाया जाएगा?
जवाब: भारत सरकार ने टीके की उपलब्धता को ध्यान में रखते हुए टीकाकरण के लिए प्राथमिकता वाले समूहों की पहचान की है। यह वे समूह हैं जिन्हें संक्रमण का सबसे ज्यादा खतरा है इसलिए इन्हें पहले टीका लगाया जाएगा। प्राथमिकता वाले इन समूहों मे पहले नम्बर पर स्वास्थ्यकर्मी और अग्रिम पंक्ति में तैनात कार्यकर्ता हैं। दूसरे नम्बर पर 50 साल से ऊपर की उम्र वाले लोग और गम्भीर बीमारियों से जूझ रहे 50 साल से कम उम्र वाले लोग शामिल हैं।
✍🏽सवाल 13- क्या कोविड का टीका लेना अनिवार्य है?
जवाब: नहीं। कोवड-19 का टीका इच्छानुसार लिया जा सकता है। हालांकि खुद को और साथ ही अपने परिवार के सदस्यों, मित्रों, सगे संबंधियों तथा सहकर्मियों को संक्रमण से बचाए रखने के लिए सभी को टीका लगवाने की सलाह दी जाती है।
✍🏽सवाल 14- टीका कितना प्रभावी और सुरक्षित है?
जवाब: कोविड का टीका लगाने की तैयारी देश में नियामक संस्थाओं द्वारा इसे मंजूरी मिलने के बाद ही की गई है।
✍🏽सवाल 15- क्या कोविड का टीका पहले से कोविड संक्रमित या संक्रमण की संभावना वाले रोगियों को दिया जा सकता है?
जवाब: ऐसे व्यक्ति के टीकाकरण स्थल पर आने से अन्य लोगों के संक्रमित होने का खतरा हो सकता है। इसलिए ऐसे व्यक्तियों को संक्रमण के लक्षण पाए जाने के 14 दिन बाद टीका लगवाना चाहिए।
✍🏽सवाल 16- क्या कोविड से स्वस्थ हो चुके व्यक्ति के लिए भी टीका लगवाना जरूरी है?
जवाब: हां। यह सलाह दी जाती है कि संक्रमण से ठीक हुए लोग भी यह टीका लगवाएं। इससे उनमें कोविड के खिलाफ मजबूत प्रतिरोधक क्षमता विकसित हो जाएगी।
✍🏽सवाल 17- कोविड के कई तरह के टीके उपलब्ध होने के बीच एक या दो टीकों को ही क्यों चुना गया?
जवाब: इन दवाओं की सुरक्षा और प्रभाव का आकलन करने के लिए कई दौर के क्लिनिकल परीक्षणों के नतीजों की जांच के बाद देश के औषध नियामक ने इन्हें लाइसेंस दिया है। हालांकि, यह सुनिश्चित किया जाना चाहिए कि टीकाकरण के पूरे चक्र के दौरान कोविड के एक ही तरह के टीके का इस्तेमाल किया जाए।
✍🏽सवाल 18- क्या देश के पास कोविड के टीकों का दो से आठ डिग्री सेल्सियस के तापमान पर भंडारण करने और एक जगह से दूसरी जगह पहुंचाने की क्षमता है?
जवाब: भारत दो करोड़ साठ लाख नवजात शिशुओं और दो करोड़ 90 लाख गर्भवती महिलाओं के लिए दुनिया का एक सबसे बड़ा टीकाकरण अभियान पहले से ही चला रहा है।
✍🏽सवाल 19. भारत में जो वैक्सीन आएगी, वह दूसरे देश जितनी असरदार होगी?
जवाब: हां, भारत में आने वाली वैक्सीन दूसरे देश जितनी ही असरदार होगी।
✍🏽सवाल 20- यह कैसे पता चलेगा कि मुझे टीका लगवाना चाहिए?
जवाब: शुरूआती चरण में स्वास्थ्य कर्मियों और अग्रिम पंक्ति वाले कार्यकर्ताओं को यह टीका लगाया जाएगा। उसके बाद टीके की उपलब्धता के आधार पर 50 वर्ष से ऊपर वाले लोगों तथा 50 वर्ष से कम उम्र के उन लोगों को यह टीका लगाया जाएगा, जो गंभीर बिमारियों से पीडि़त हैं। लाभार्थियों को टीका किस जगह, किस समय और किस दिन लगाया जाएगा इसकी जानकारी उनके पंजीकृत मोबाइल नम्बर पर भेज दी जाएगी। लाभार्थियों के पंजीकरण और टीकारण में किसी तरह की दिक्कत न आए इसके लिए यह व्यवस्था की गई है।
✍🏽सवाल: 21- क्या लाभार्थी के लिए पंजीकरण कराते समय अपना फोटो/फोटो पहचान-पत्र देना जरूरी होगा?
जवाब: लाभार्थी को पंजीकरण कराते समय अपना फोटो पहचान पत्र देना जरूरी होगा, ताकि टीकाकरण के समय उसका सत्यापन किया जा सके।
✍🏽सवाल: 22- टीकाकरण स्थल पर लोगों के लिए किस तरह की सावधानी बरतना जरूरी है?
जवाब: टीका लगवाने के लिए आने वाले लोगों को टीकाकरण स्थल पर कम से कम आधा घंटा पहले पहुंचना होगा।
टीका लगवाने के बाद किसी तरह की परेशानी महसूस होने पर समीप के स्वास्थ्य अधिकारियों/एएनएम और आशा कर्मियों को इसकी जानकारी दी जा सकती है।
टीकाकरण स्थल पर सुरक्षित दूरी, मास्क पहनने और हाथों की सफाई का पूरा ध्यान रखना होगा।
✍🏽सवाल 23- कोविड के टीके के दुष्प्रभाव की कितनी संभावना है?
जवाब: अन्य टीकों के समान ही इसमें भी टीका लगाए जाने के बाद किसी भी व्यक्ति को हल्का बुखार और दर्द आदि की शिकायत हो सकती है।
राज्यों से कहा गया है कि वे टीकाकरण स्थल पर ऐसी किसी भी स्थिति से निपटने के लिए पहले से सभी व्यवस्था करके रखें।









Bharti