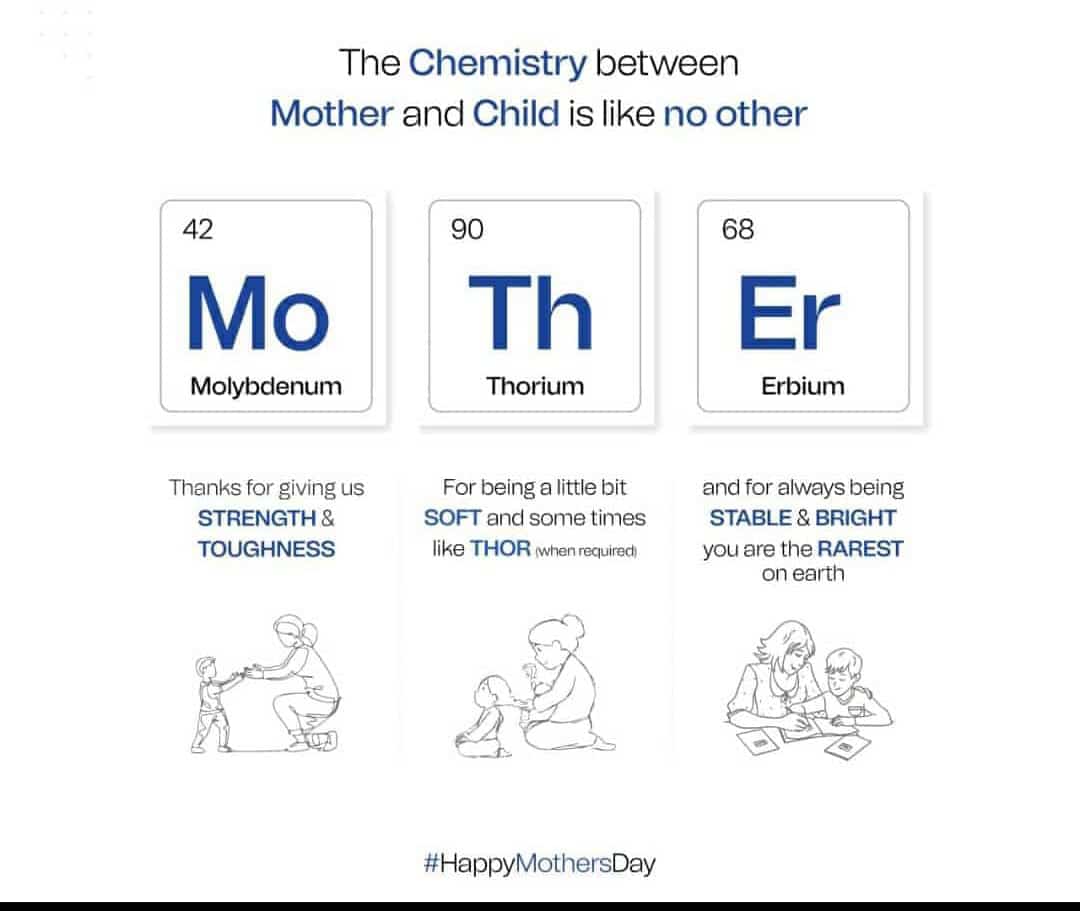
Today I went to Chinmaya mission to attend mother's day festival.There was a program that had a pooja (in which you place flowers at your mother's feet)and a cultural program with lots of skits and songs,it was very fun to watch and I sang a Maa Laxmi bhajan and also concluding prayers. There was also a star wars skit. After the cultural program there was a cookout in which all the dads made lots and lots of food. I ate 2 veggie burgers, 2 bowls of ice cream,A bhatura (it is like a puffed tortilla) and a dosa (it is a Indian food).There was also Mexican food and vadas. In one stall there were photos for sale.I think there were about 1000 people.I ate on top of a hill and it was very warm outside.There was a acharya(Swapnaji) who did a talk on respecting your mother.The summary of the talk was to get up in the morning and make your bed,then get ready for school and eat a good breakfast,go to school,come home and do your homework without your mom telling you to do it.There was lots of parking space, many cars were parked on the hill and some on roadside.It was fun overall.Now I am going for a Nerf war in Skinner park.And probably after that I will go to Caden's house for a play date.
Om Raizada
.jpg)
.jpg)
.jpg)
.jpg)
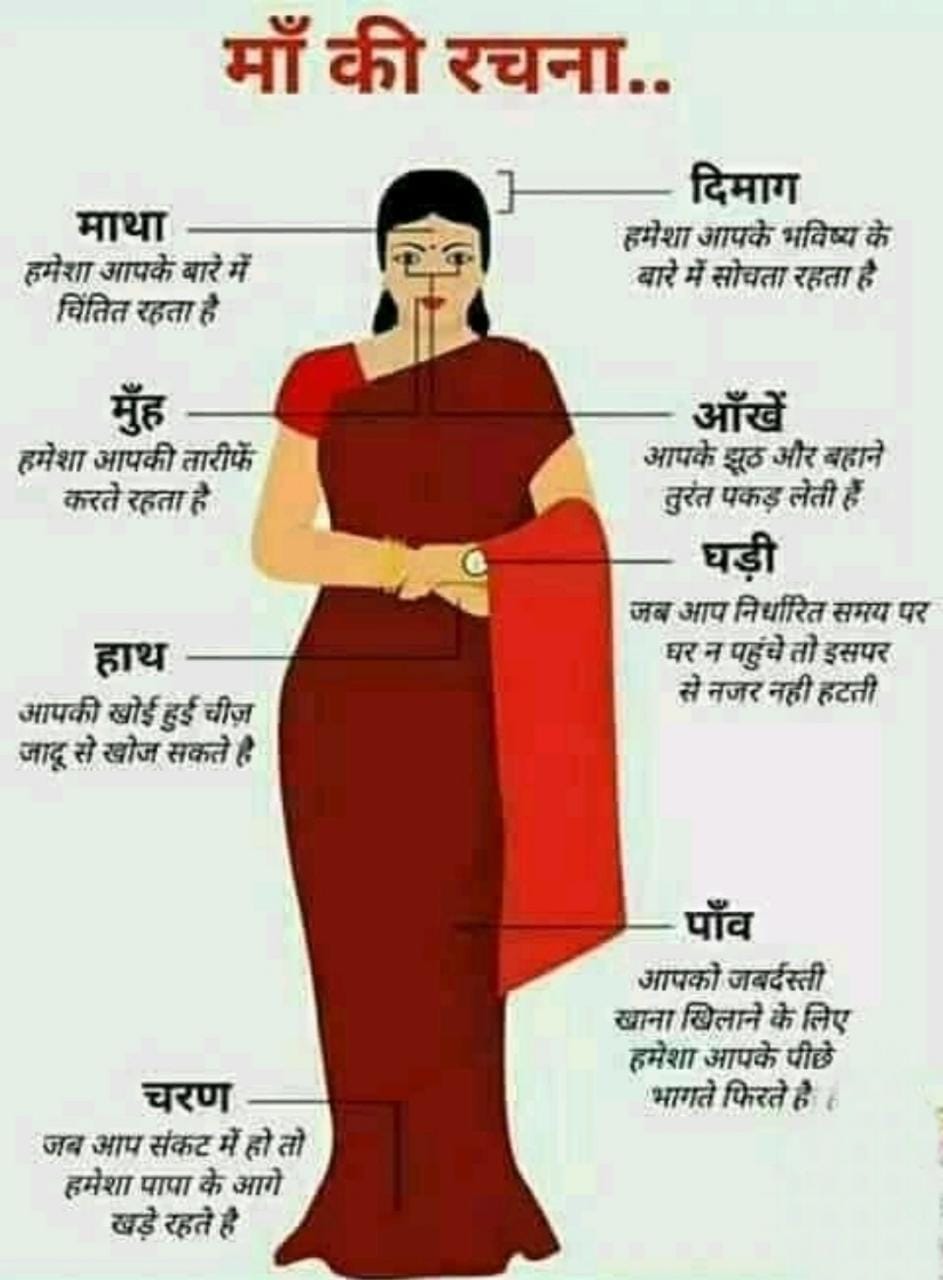
माँ का पल्लू
मुझे नहीं लगता कि आज के बच्चे यह जानते हो कि पल्लू क्या होता है, इसका कारण यह है कि आजकल की माताएं अब साड़ी नहीं पहनती हैं। पल्लू बीते समय की बातें हो चुकी है।
माँ के पल्लू का सिद्धांत माँ को गरिमा मायी छवि प्रदान प्रदान करने के लिए था।
इसके साथ ही, यह गरम बर्तन को चूल्हा से हटाते समय गरम बर्तन को पकड़ने के काम भी आता था।
-पल्लू की बात ही निराली थी। पल्लू पर तो कितना भी लिखा जा सकता है ---- -
पल्लू बच्चों का पसीना / आँसू पोंछने, गंदे कानों/मुह की सफाई के लिए भी इस्तेमाल किया जाता था। माँ इसको अपना हाथ पोंछने के लिए तौलिया के रूप में भी इस्तेमाल का लेती थी । खाना खाने के बाद पल्लू से मुंह साफ करने का अपना ही आनंद होता था।
-- कभी आँख मे दर्द होने पर माँ अपने पल्लू को गोल बनाकर, फूँक मारकर, गरम करके आँख में लगा देतीं थी , सब दर्द उसी समय गायब हो जाता था ।
-माँ की गोद में सोने वाले बच्चों के लिए उसकी गोद गद्दा और उसका पल्लू चादर का काम करता था ।
-- जब भी कोई अंजान घर पर आता , तो बच्चा उसको माँ के पल्लू की ओट ले कर देखता था । जब भी बच्चे को किसी बात पर शर्म आती, वो पल्लू से अपना मुंह ढक कर छुप जाता था ।
-- जब बच्चों को बाहर जाना होता , तब माँ का पल्लू एक मार्गदर्शक का काम करता था । जब तक बच्चे ने हाथ में पल्लू थाम रखा होता, तो सारी कायनात उसकी मुट्ठी में होती थी ।
-- जब मौसम ठंडा होता था , मां उसको अपने चारो ओर लपेट कर ठंड से बचने की कोशिश करती ।
पल्लू एप्रन का काम भी करता था । पल्लू का उपयोग पेड़ों से गिरने वाले जामुन और मीठे सुगंधित फूलों को लाने के लिए किया जाता था। पल्लू घर मे रखे समान से धूल हटाने मे भी बहुत सहायक होता था ।
पल्लू में गांठ लगा कर माँ एक चलता फिरता बैंक या तिजोरी रखती थी और अगर सब कुछ ठीक रहा तो कभी कभी उस बैंक से कुछ पैसे भी मिल जाते थे।
मुझे नहीं लगता की विज्ञान इतनी तरक्की करने के बाद भी पल्लू का विकल्प ढूंढ पाया है ।
पल्लू कुछ और नहीं बल्कि एक जादुई एहसास है । पुरानी पीढ़ी से संबंध रखने वाले
अपनी माँ के इस प्यार और स्नेह को हमेशा महसूस करते हैं, जो कि आज की पीढ़ियों की समझ से शायद गायब
मां के रहते इस जीवन में,गम न कोई होता है।
साथ न दे बेसक कोई,मां का प्यार तो मिलता है।
मां ममता का सागर है,और त्याग कुछ रिश्तों का।
मां से बड़ा न जग में कोई,रिश्ता है अहसासों का।
संतान भले हो गर्दिश में,मां का आँचल मिलता है।
साथ न दे बेसक कोई,मां का प्यार तो मिलता है।
नौ महीना बच्चे के ख़ातिर,कष्ट बहुत उठाती है।
तब इस संसार की एक मां,रचना कर पाती है।
जग जननी है मां,ये संसार उसी से चलता है।
साथ न दे बेसक कोई,मां का प्यार तो मिलता है।
मां ईश्वर का रूप यहां,और मां से बड़ा नही कोई।
जिन बच्चों की माएं नही,उनका जग में कोई नही।
दर-दर खाते ठोकर वो,प्यार न उनको मिलता है।
साथ न दे बेसक कोई,मां का प्यार तो मिलता है।
हे मित्र आरजू आशू की,आंखों से आंशू बहे नही।
सेवा हो मां की हरदम,मां को रुलाना कभी नही।
चोट लगी यदि मां को तो,ईश्वर भी तब रोता है।
साथ न दे बेसक कोई,मां का प्यार तो मिलता है।

























किसी की ख़ातिर अल्ला होगा, किसी की ख़ातिर राम
लेकिन अपनी ख़ातिर तो है, माँ ही चारों धा
जब आँख खुली तो अम्मा की गोदी का एक सहारा था
उसका नन्हा-सा आँचल मुझको भूमण्डल से प्यारा था
उसके चेहरे की झलक देख चेहरा फूलों-सा खिलता था
उसके स्तन की एक बूंद से मुझको जीवन मिलता था
हाथों से बालों को नोचा, पैरों से खूब प्रहार किया
फिर भी उस माँ ने पुचकारा हमको जी भर के प्यार किया
मैं उसका राजा बेटा था वो आँख का तारा कहती थी
मैं बनूँ बुढ़ापे में उसका बस एक सहारा कहती थी
उंगली को पकड़ चलाया था पढ़ने विद्यालय भेजा था
मेरी नादानी को भी निज अन्तर में सदा सहेजा था
मेरे सारे प्रश्नों का वो फौरन जवाब बन जाती थी
मेरी राहों के काँटे चुन वो ख़ुद ग़ुलाब बन जाती थी
मैं बड़ा हुआ तो कॉलेज से इक रोग प्यार का ले आया
जिस दिल में माँ की मूरत थी वो रामकली को दे आया
शादी की, पति से बाप बना, अपने रिश्तों में झूल गया
अब करवाचौथ मनाता हूँ माँ की ममता को भूल गया
हम भूल गए उसकी ममता, मेरे जीवन की थाती थी
हम भूल गए अपना जीवन, वो अमृत वाली छाती थी
हम भूल गए वो ख़ुद भूखी रह करके हमें खिलाती थी
हमको सूखा बिस्तर देकर ख़ुद गीले में सो जाती थी
हम भूल गए उसने ही होठों को भाषा सिखलाई थी
मेरी नींदों के लिए रात भर उसने लोरी गाई थी
हम भूल गए हर ग़लती पर उसने डाँटा-समझाया था
बच जाऊँ बुरी नज़र से काला टीका सदा लगाया था
हम बड़े हुए तो ममता वाले सारे बन्धन तोड़ आए
बंगले में कुत्ते पाल लिए माँ को वृद्धाश्रम छोड़ आए
उसके सपनों का महल गिरा कर कंकर-कंकर बीन लिए
ख़ुदग़र्ज़ी में उसके सुहाग के आभूषण तक छीन लिए
हम माँ को घर के बँटवारे की अभिलाषा तक ले आए
उसको पावन मंदिर से गाली की भाषा तक ले आए
माँ की ममता को देख मौत भी आगे से हट जाती है
गर माँ अपमानित होती, धरती की छाती फट जाती है
घर को पूरा जीवन देकर बेचारी माँ क्या पाती है
रूखा-सूखा खा लेती है, पानी पीकर सो जाती है
जो माँ जैसी देवी घर के मंदिर में नहीं रख सकते हैं
वो लाखों पुण्य भले कर लें इंसान नहीं बन सकते हैं
माँ जिसको भी जल दे दे वो पौधा संदल बन जाता है
माँ के चरणों को छूकर पानी गंगाजल बन जाता है
माँ के आँचल ने युगों-युगों से भगवानों को पाला है
माँ के चरणों में जन्नत है गिरिजाघर और शिवाला है
हिमगिरि जैसी ऊँचाई है, सागर जैसी गहराई है
दुनिया में जितनी ख़ुशबू है माँ के आँचल से आई है
माँ कबिरा की साखी जैसी, माँ तुलसी की चौपाई है
मीराबाई की पदावली ख़ुसरो की अमर रुबाई है
माँ आंगन की तुलसी जैसी पावन बरगद की छाया है
माँ वेद ऋचाओं की गरिमा, माँ महाकाव्य की काया है
माँ मानसरोवर ममता का, माँ गोमुख की ऊँचाई है
माँ परिवारों का संगम है, माँ रिश्तों की गहराई है
माँ हरी दूब है धरती की, माँ केसर वाली क्यारी है
माँ की उपमा केवल माँ है, माँ हर घर की फुलवारी है
सातों सुर नर्तन करते जब कोई माँ लोरी गाती है
माँ जिस रोटी को छू लेती है वो प्रसाद बन जाती है
माँ हँसती है तो धरती का ज़र्रा-ज़र्रा मुस्काता है
देखो तो दूर क्षितिज अंबर धरती को शीश झुकाता है
माना मेरे घर की दीवारों में चन्दा-सी मूरत है
पर मेरे मन के मंदिर में बस केवल माँ की मूरत है
माँ सरस्वती, लक्ष्मी, दुर्गा, अनुसूया, मरियम, सीता है
माँ पावनता में रामचरितमानस् है भगवद्गीता है
अम्मा तेरी हर बात मुझे वरदान से बढ़कर लगती है
हे माँ तेरी सूरत मुझको भगवान से बढ़कर लगती है
सारे तीरथ के पुण्य जहाँ, मैं उन चरणों में लेटा हूँ
जिनके कोई सन्तान नहीं, मैं उन माँओं का बेटा हूँ
हर घर में माँ की पूजा हो ऐसा संकल्प उठाता हूँ
मैं दुनिया की हर माँ के चरणों में ये शीश झुकाता हूँ
By Dr. Sunil Jogi
NIH NLM LogoLog in
Access keysNCBI HomepageMyNCBI HomepageMain ContentMain Navigation
Search PMC Full-Text Archive
Search PMC Full-Text Archive
Search in PMC
Run this search in PubMed
Advanced Search User Guide
Journal List Obstet Med v.1(2); 2008 Dec PMC4989712
Logo of obm
Obstet Med. 2008 Dec; 1(2): 56–64.
Published online 2008 Dec 1. doi: 10.1258/om.2008.080008
PMCID: PMC4989712
PMID: 27582787
Fetal microchimerism and maternal health during and after pregnancy
Keelin O'Donoghue, PhD MRCOG
Author information Article notes Copyright and License information Disclaimer
Go to:
Abstract
Trafficking of fetal cells into the maternal circulation begins very early in pregnancy and the effects of this cell traffic are longlasting. All types of fetal cells, including stem cells, cross the placenta during normal pregnancy to enter maternal blood, from where they may be recovered in pregnancy for the purpose of genetic prenatal diagnosis. Fetal cells can also be located in maternal tissues during and after pregnancy, and persist as microchimeric cells for decades in marrow and other organs. Although persistent fetal cells were first implicated in autoimmune disease, subsequent reports routinely found microchimeric cells in healthy tissues and in non-autoimmune disease. Parallel studies in animal and human pregnancy now suggest instead that microchimeric fetal cells play a role in the response to tissue injury. However, it is still not clear whether microchimeric fetal cells persisting in the mother are an incidental finding, are naturally pathogenic or act as reparative stem cells, and the environmental or biological stimuli that determine microchimeric cell fate are as yet undetermined. Future studies must also focus on investigating whether fetal cells create functional improvement in response to maternal injury and whether this response can be manipulated.
The pregnancy-acquired low-grade chimeric state of women could have far-reaching implications, influencing recovery after injury or surgery, ageing, graft survival after transplantation, survival after cancer as well as deciding the protective effect of pregnancy against diseases later in life. Lifelong persistence of fetal cells in maternal tissues may even explain why women live longer than men.
Keywords: autoimmune disease, fetal microchimerism, fetal stem cells, pregnancy, tissue repair
Go to:
FETOMATERNAL CELL TRAFFICKING AND THE DISCOVERY OF PERSISTING FETAL CELLS
Many events occur during pregnancy and fetal development, which not only influence the health of a child into adulthood, but also have long-term effects on the health of the mother.1 One such event is the passage of fetal cells into the maternal circulation.
Small numbers of fetal cells traffic across the placenta in every pregnancy.2 Trophoblasts were the first cell type found in maternal blood, whereas other cell types come from the fetal bloodstream.3,4 The presence of fetal erythroblasts, leukocytes, haemopoietic and mesenchymal stem cells (MSC) in maternal blood is not surprising given apposition of fetomaternal placental vasculature. Frequent histological defects in trophoblast continuity and identification of intervillous thrombi comprising mixed maternal and fetal cells, points to the mechanism being fetomaternal haemorrhage from microtrauma.5 Fetal cells in maternal blood have been investigated for non-invasive prenatal diagnosis for over 30 years, with research focussing on cells with limited lifespan to avoid persistence from previous pregnancies.2 Persistence probably explains occasional male lymphocytes found in peripheral blood of women giving birth to girls. However, Bianchi et al. 6 reported in 1996 male fetal haemopoietic stem cells (HSC) in the bloodstream of 6/8 women delivered of a boy up to 27 years before, which established the field of fetal microchimerism.
Go to:
WHAT IS FETAL MICROCHIMERISM?
A chimera is an animal that has two or more different populations of genetically distinct cells and was named after the mythological creature, chimera.7 Microchimerism is the existence of a low-level allogeneic cell population within a host. Microchimeric states can arise spontaneously, such as in dizygotic twins with connected placental vasculature or iatrogenically, where microchimerism arises after blood transfusion, stem cell or organ transplantation.8,9 Fluorescence in situ hybridization (FISH) has demonstrated sex-mismatched donor cells in host tissues after transplantation and conversely, sex-mismatched host cells have been demonstrated in solid donor organs. Although microchimerism has been documented decades after transplantation, its significance is uncertain as graft versus host disease (GVHD) is a chimeric condition, while microchimeric cells can help establish donor tolerance to the graft.8 Pregnancy is another source of microchimeric cells, implicated by studies in women identifying Y-chromosomes from a male fetus. Male and female fetal cells cross the placenta in equal numbers, but studies of microchimerism rely on the demonstration of the Y chromosome as proof of principle.
Fetal microchimerism is defined as low levels of fetal cells harbouring in maternal blood and tissues during and for years after pregnancy. It has been proposed as ‘a state of balance between host versus graft and graft versus host reactions, leading to the acceptance of the allogeneic fetus’.10 Presumably, the placental immune suppression that is needed to maintain the allogeneic pregnancy also helps establish microchimerism. This immune suppression of pregnancy may remain for several months after delivery, allowing persisting fetal cells time to establish themselves.3 All parous women thus become chimeric.
Go to:
THE AUTOIMMUNE DISEASE HYPOTHESIS
Nelson et al. 11 who speculated that fetal microchimerism mediated the higher prevalence of autoimmune disease in women, first made the connection between fetal microchimerism and human pathology. This hypothesis is supported by similarities of chronic GVHD to some autoimmune conditions, their predilection for women of childbearing age with an increased incidence after the reproductive years, and experimental models of autoimmune disease showing fetal cells in affected tissue.12–14 Microchimeric fetal cells have now been demonstrated in tissues from women with systemic sclerosis, primary biliary cirrhosis, Sjogren's syndrome, rheumatoid arthritis, systemic lupus erythaematosus (SLE) and autoimmune thyroid diseases.4
Fetal microchimerism has been firmly implicated in the pathogenesis of systemic sclerosis based on studies confirming increased microchimerism in peripheral blood, skin lesions and other organs compared with controls, and the identification of higher numbers of fetal cells in unaffected skin from affected patients compared with controls, as well as clinical similarities with GVHD.14,15 However, the autoimmune hypothesis is weakened by the discovery of persistent fetal cells in non-autoimmune conditions such as hepatitis C and thyroid adenomas and by the many reports of fetal microchimeric cells in healthy tissues.16 Studies suggest that most autoimmune conditions are not significantly associated with more microchimeric cells in blood or tissues when compared with controls (Table 1). In addition, although fetal cells appear to accumulate in clinically affected organs,17 there is no conclusive evidence that fetal cells cause autoimmune disease. Thus, it cannot be concluded that fetal microchimerism always results in a graft versus host phenomenon.18,19
Table 1
Analysis of all published studies of microchimerism in diseased and healthy tissues in pregnant and postreproductive women over a 10-year period
Disease Tissues studied Type of controls Method Controls Cases
n % + n % +
Systemic sclerosis Peripheral blood14 Healthy women PCR 25 4 69 46
Skin14 Osteoarthritis PCR 68 0 19 58
Peripheral blood51 Healthy women PCR 48 33 20 60
Peripheral blood52 Healthy women PCR 16 25 17 58
Salivary glands53 None PCR – – 11 45
Peripheral blood54 Healthy women PCR 20 30 20 40
Peripheral blood55 Healthy women PCR 41 20 20 50
Diseased organs15 Postmortem tissues FISH 3 0 5 100
Skin56 Healthy women PCR 57 35 49 28
Peripheral blood57 Healthy women PCR 40 5 47 8.5
Skin36 Healthy women PCR, FISH 10 0 5 100
SLE Diseased organs17 None FISH – – 1 100
Peripheral blood58 Healthy women PCR 18 33 28 68
Peripheral blood59 Healthy women PCR 24 50 22 50
Skin60 Other skin disease FISH 4 0 6 0
Diseased organs33 Healthy women FISH 34 14 7 50
Sjogren's syndrome Peripheral blood61 Healthy women PCR 18 11 30 0
Salivary glands62 Healthy women PCR 3 0 6 0
Peripheral blood63 Healthy women PCR 18 0 20 0
Peripheral blood64 Healthy women PCR 20 25 23 13
Salivary glands64 Other disease PCR 8 13 20 55
Salivary glands65 Healthy women PCR 10 0 28 36
Salivary glands53 None PCR – – 16 0
Primary biliary cirrhosis Liver66 Hepatitis C FISH 3 0 10 0
Liver67 Healthy women PCR 39 72 37 70
Peripheral blood68 Healthy women PCR 36 31 36 36
Peripheral blood69 Healthy women PCR 18 0.05 18 0
Liver69 Other liver disease FISH 20 0 19 42
Peripheral blood70 Healthy women PCR 20 25 20 45
Liver70 Other liver disease PCR 25 32 15 33
Liver71 Other liver disease PCR, FISH 52 4 28 18
Hashimoto's disease Thyroid72 Nodular goitre PCR 25 4 17 47
Thyroid37 Other thyroid disease FISH 25 60 6 83
Thyroid73 Other thyroid disease FISH 9 20 25 60
Thyroid74 Healthy thyroid PCR 17 0 21 38
Grave's disease Peripheral blood75 Healthy women PCR 30 13 17 47
Thyroid75 Other thyroid disease PCR 6 0 20 20
Thyroid73 Other thyroid disease FISH 9 20 15 40
PEP Skin46 Healthy women PCR 26 0 10 60
Lichen planus Oral mucosa76 Healthy women FISH 4 0 15 0
Oral mucosa77 None FISH 0 0 12 0
Lichen sclerosus Vulval skin78 Other vulval disease PCR 27 37 27 40
Vulval skin79 Healthy tissue PCR, FISH 6 0 15 0
Hepatitis C Liver35 None FISH – – 1 100
Liver71 Other liver disease PCR, FISH 52 4 25 8
Liver80 Other liver disease PCR, FISH 7 43 10 70
Pneumonitis Peripheral blood81 Healthy women FISH 43 16 103 33
Cervical cancer Cervical tissue44 Healthy women FISH 4 0 8 100
Breast cancer Peripheral blood43 Healthy women PCR 29 48 22 14
Lung cancer Lung tumour34 None FISH, PCR 0 0 7 57
Numbers of controls or cases investigated (n) 20 (0–68) 19 (0–103)
Detection of microchimerism (%) 8 (0–72) 41 (0–100)
Open in a separate window
Median numbers of controls investigated per study was 20 (range 0–68) and median numbers of cases examined was 19 (range 0–103). Microchimerism was detected in fewer controls (median 8%, range 0–72) than in diseased blood and tissues (median 41%, range 0–100) *Denotes women selected for a history of male pregnancies += positive male cell microchimerism. SLE = systemic lupus erythaematosus, PEP = polymorphic eruption of pregnancy, PCR = polymerase chain reaction, FISH = fluorescence in situ hybridization
Go to:
FETAL MICROCHIMERISM OCCURS IN HEALTHY WOMEN
The frequency of fetal microchimerism in healthy women is unknown, but controls studied in the autoimmune disease reports suggest wide variation (median 8%, range 0–72%) (Table 1). Male DNA was detected in 29% of apheresis products from non-pregnant female marrow donors and in 48% of the enriched HSC fraction, confirming chimerism is common.20 All mice in a study many years ago demonstrated fetal cells from an earlier pregnancy persisting in lymphoid tissue,10 while more recently Khosrotehrani et al. 21 used murine models to show microchimerism in a variety of tissues in all pregnant mice during pregnancy.
Fetal microchimerism in humans seems as frequent as in animal studies and may even be always present. This was confirmed by a report of male presumed-fetal cells found in bone marrow and bone from all postreproductive women who had sons decades earlier, but none in those without sons (Figure 1). These data showed that fetal cells transferred into maternal blood during pregnancy engrafted and persisted long term in marrow and bone, but also implied the fetal cells involved might be MSC.22
An external file that holds a picture, illustration, etc.
Object name is 10.1258_om.2008.080008-fig1.jpg
Figure 1
Male fetal microchimerism in female marrow, bone, lung and appendix. A section of rib was collected to obtain marrow (a), with adherent cell cultures (b) and sections of bone (c) analysed for the presence of the Y chromosome using XY-Fluorescence in situ hybridisation (FISH). A section of lung tumour (adenocarcinoma, d) was obtained at the same surgery from this cohort of postreproductive women. Sections of appendix were obtained from pregnant women undergoing clinically indicated appendicectomy (e, f). The X chromosomes in (d) and (e) are labelled with SpectrumOrange™, with the Y chromosomes labelled (d, e; arrows) with SpectrumGreen™. The Y chromosomes in (b, c and f) were identified using a Y FISH probe, labelled with SpectrumOrange™ (arrows, red signals). A male (red signal) CD3-positive (FITC, green) lymphocyte is shown in (f). Magnification ×100
Go to:
WHAT INFLUENCES MATERNAL DEVELOPMENT OF FETAL MICROCHIMERISM?
Factors predisposing to the development of fetal microchimerism are much debated. There is more fetomaternal cell trafficking where the placenta is abnormal, after invasive diagnostic procedures and in certain complications of pregnancy, such as fetal aneuploidy, pregnancy loss or termination and pre-eclampsia,18 which implies that more microchimerism should be established. However, early fetal loss seems the only pregnancy complication significantly influencing microchimerism. Khosrotehrani et al. 23 also speculated that miscarriage allowed more primitive types of fetal cells with the greater capacity to differentiate to enter the maternal circulation.
The HLA relationship between maternal and fetal cells appears relevant in determining the long-term effects of fetal cell trafficking and persistence.24 HLA-incompatible fetuses may be at an advantage in pregnancy and HLA-compatibility seems to confer a greater risk of autoimmune disease after pregnancy. For example, in studies of rheumatoid arthritis, fetomaternal HLA disparity is associated with pregnancy-induced remission of disease, whereas women with systemic sclerosis are almost nine times more likely to have given birth to a HLA-compatible child.25 However, although certain maternal HLA alleles are more frequently associated with fetal microchimerism,11 this finding is controversial.26,27 Genetic and environmental factors are also likely to contribute to the effects of microchimerism, possibly by regulating the proliferation or differentiation of the fetal cells.24
Go to:
FETAL MICROCHIMERIC CELLS AND THE REPAIR OF MATERNAL TISSUES
In recent years, it has been suggested that microchimeric fetal cells respond to maternal tissue injury and that the primary biological role of fetal microchimerism is reparative.26 Injured tissue might produce signals that mobilize fetal stem cells from the pregnancy reservoir or the local microenvironment might stimulate the differentiation of engrafted stem cells into specialized cells.3,26 It is also possible that chronic tissue injury might actually promote the establishment of microchimerism. Christner et al. 28 used murine models of skin fibrosis to show that fetal cells were recruited to sites of injury and other rodent work also implicates fetal microchimerism in tissue repair rather than disease pathogenesis.29,30 In women, male cells were found in a high proportion of those with hepatitis C, thyroid adenomas and polymorphic eruption of pregnancy (PEP). However, the first direct association between fetal microchimerism and tissue repair in humans was Khosrotehrani's demonstration of fetal cells bearing epithelial, leukocyte and hepatocyte markers in diseased tissue, which were absent from male cells in adjacent healthy tissue.31,32
Recently, microchimerism in SLE has been re-investigated to determine any association between the distribution of microchimerism in affected tissues and the amount of injury. Male presumed-fetal cells were identified significantly more often in diseased organs from women with SLE than in controls, but were also more common in organs that experienced a non-SLE-related injury.33 This confirms a relationship between microchimerism and tissue injury rather than an association with autoimmune disease alone.
Go to:
IS A FETAL STEM CELL RESPONSIBLE FOR MICROCHIMERISM?
The fetal cell type responsible for microchimerism is unknown and candidates include all cells in fetal blood that persist long term.3,18 Several lines of evidence support a stem cell being involved. First, infused stem cells migrate to host tissues and differentiate to adopt characteristics of host organs. Second, allogeneic fetal cells proliferate in maternal bone marrow during index and subsequent pregnancies.10 Next, persistent fetal cells are found among stem cell populations decades after pregnancy.6 Finally, differentiated male cells become indistinguishable from female tissue years after pregnancy, suggesting fetal cells expand within host tissue, while microchimeric fetal cells bear a variety of different lineage markers.3,22,34
The fact that fully differentiated fetal cells with a short half-life and no self-renewal ability appear regularly in maternal tissues years after pregnancy is substantial evidence for fetal stem cell involvement in microchimerism.3 In support of this theory, Johnson et al. 35 showed that male cells in a patient's liver came from a pregnancy terminated 17–19 years beforehand, while O'Donoghue's et al. 22 work in bone marrow and Sawaya's in skin showed fetal microchimerism in women decades after the birth of their last son.36 The demonstration of entirely male sections of thyroid, identical to surrounding female cells, also suggested that this woman had acquired a male stem cell during her pregnancy and that the stem cell had migrated to her thyroid to form new thyroid cells.37
The nature of the stem cell involved is controversial, but could be mesenchymal, haemopoietic or a common precursor.34 Bianchi's group acknowledged this uncertainty, adopting the term pregnancy-associated progenitor cells.18,26,37 The literature on fetomaternal haemopoietic traffic and the original findings of persistent cells with haemopoietic markers implicates HSC.6,18 However, fetal MSC may also be involved.22,34 MSC express numerous adhesion molecules that, along with their adherent properties, suggest they readily implant in tissues. Adult marrow-derived MSC engraft widely in animal models and preferentially home to bone marrow. Next, MSC are immunomodulatory and non-immunogenic, which may aid ability to persist in tissues. Finally, male cells identified in bone marrow from women with sons were immunophenotypically MSC.3,22
Go to:
INSIGHTS FROM MURINE MODELS OF MICROCHIMERISM
Human studies on microchimerism are limited by ethical constraints, the necessary use of archived tissues and by incomplete reproductive histories. For these reasons, some groups have turned to using murine models of disease (Table 2).18 Further, the many transgenic murine lines available, along with their short gestation have enabled the use of markers to track fetal cells in the mother mouse.
Table 2
Key findings in rodent studies of fetal microchimerism
Model Tissues studied Disease process involved Findings
CBA/HT6T6 mice10 Lymph nodes, marrow, spleen GVHD induced in healthy mice
Microchimerism detected during pregnancy
Microchimerism increased in subsequent pregnancy
BALB/cJ retired breeder mice28 Peripheral blood, skin Vinyl chloride injection to mimic systemic sclerosis
Microchimerism increased 48-fold after injections to induce dermal fibrosis
Transgenic GFP mice (Tg immunized)30 Thyroid, marrow, peripheral blood Autoimmune thyroiditis
Microchimeric fetal cells identified in maternal thyroid during and after pregnancy
Frequency highest in thyroid compared to blood or marrow
Transgenic GFP mice21 Liver, spleen, kidney, heart, brain, marrow Healthy
Microchimerism detected in all tissues during pregnancy
Frequency highest in fetal lungs
Microchimerism undetectable three weeks after first delivery
Transgenic GFP rats29 Peripheral blood, liver, kidney Ethanol liver damage Gentamicin kidney damage
Microchimerism detected in multiple organs
Microchimeric cells were hepatocytes and renal tubular cells
Transgenic GFP mice38 Liver, spleen CCl4 injection
Microchimeric fetal cells detectable in liver and spleen after chemical injury only
Partial hepatectomy
Fetal cell number increased over time after injury
Transgenic GFP mice39 Brain Excito-toxic brain injury
Microchimeric fetal cells detected in maternal brain
Number of fetal cells increased after brain injury
Fetal cells clustered at sites of injury
Transgenic GFP mice (VEGF-2 promoter)40 Skin Hypersensitivity skin reactions
Microchimeric fetal cells located in inflamed skin in greater number than non-inflamed skin
Fetal cells homed to inflamed skin and formed new endothelium
Open in a separate window
Khosrotehrani found that GFP+ microchimeric fetal cells which were present in all tissues tested in pregnant mice, declined in frequency with increasing time postpartum and were still present in some retired breeders.21 This study also confirmed that the natural history of microchimerism in the mouse was equivalent to the human. Christner first showed that fetal cells were recruited to sites of injury in response to tissue damage: injection of vinyl chloride in retired breeders showed a huge increase in male genetic material in maternal peripheral blood and a doubling in skin fibrosis not seen in controls.28 As in humans, there are now increasing data to show that fetal microchimerism increases in injured rodents (Table 2).38 Chemical hepatic and renal damage in rats recruited fetal cells to the injury site with tissue-specific differentiation.29 Fetal cells in maternal brain doubled after neural injury39 and, in another model, greater numbers of fetal cells were demonstrated in inflamed skin compared with non-inflamed areas.40
Murine models of microchimerism have also been successfully used to track fetal cells postpartum, using bioluminescence of the living animal.18,26,40,41 When wild-type mothers with fetuses transgenic for luciferase driven by a collagen promoter were monitored using bioluminescence after skin biopsy, luciferase activity was detected in vivo in maternal excisional wounds one week after injury, which then diminished with healing to be undetectable postpartum. Fresh maternal injury postpartum resulted in the skin activation of the fetal collagen promoter, confirming the microchimeric cells as fetal and suggesting their non-haemopoietic origin.41
Go to:
THE HIDDEN LINK BETWEEN PREGNANCY AND CANCER
In a recent study, fetal microchimerism was investigated in healthy and diseased lung tissues from a group of women undergoing surgery for suspected lung cancer.34 These women formed part of the cohort who donated marrow and rib sections, and their obstetric histories were known.22 Male cells were identified using FISH and polymerase chain reaction techniques in pathological lung tissue from all women with known male pregnancies, but not in those without sons (Figure 1). The frequency of male microchimeric cells was seven-fold greater in lung tumours than marrow and was two-fold greater than in normal bone from the same women. Male cells in lung were clustered in tumour rather than in surrounding healthy tissues.34 This not only implies that fetal cells transferred into maternal blood during pregnancy engraft marrow and can be located years later in other organs, but also implicates fetal microchimeric cells in tissue repair. Although the authors could not prove the origin of the microchimeric cells, they suggested that fetal cells present at sites of injury were stem cells, possibly recruited from bone marrow.34
Women with lung cancer have better survival rates than men, which cannot be easily explained by cancer stage, type or treatment. Reproductive factors have been suggested as the reason for this disparity,42 but as nulliparous women have been shown to have a worse prognosis than those with children, it has been speculated that persistent fetal microchimeric cells might also play a role in survival.34 Breast cancer is more common in the nulliparous woman and it has also been suggested that microchimeric fetal cells play a role in the protective effect of parity. In support of this, Gadi and Nelson43 recently reported peripheral blood microchimerism significantly more often in healthy women compared with women with breast cancer. They proposed that naturally acquired allogeneic fetal cells provided protection against cancer antigens through priming of the maternal immune system during pregnancy.43 Few other reports have examined fetal microchimerism in relation to cancer. A study of thyroid microchimerism included seven women with thyroid carcinoma, of whom three of four with sons had male cells detected,37 while male cells with epithelial markers were found in cervical cancers from five of eight women with sons, suggesting that microchimeric cells might play a role in carcinogenesis.34,44
In chronic injury, it is speculated that the recruitment of stem cells from bone marrow might transform into cancer over time.45 Alternatively, damaged tissue releases chemokines, which help recruit stem cells, and it is biologically plausible that engrafted fetal stem cells could be also attracted. In the lung tumour study, microchimerism was also established in comparatively greater numbers in cancerous tissues than in healthy tissues. However, microchimeric cells were clustered in benign as well as malignant lung tumours, which does not support the idea that such cells are preferentially involved in malignancy, but suggests instead they are involved in the injury and repair process.34
Go to:
FETAL MICROCHIMERISM AND TISSUE REPAIR DURING HUMAN PREGNANCY
For years, the only evidence that microchimeric cells engraft during human pregnancy came from male DNA in skin biopsies from women with PEP, a cutaneous eruption of unknown pathogenesis, which occurs during the third trimester of pregnancy and disappears after delivery. Male fetal cells were identified in skin biopsies from pregnant women with PEP and investigators hypothesized that fetal cells migrated to the maternal skin during pregnancy to cause an inflammatory response, leading to the disease.46
A novel study recently used the appendix as a model of injured tissue to determine whether fetal cells participated in tissue repair during pregnancy.32 Male cells were found in appendix specimens from all women with sons, who underwent clinically indicated appendicectomy in pregnancy (Figure 1). Preliminary analysis implied that women with histologically confirmed acute appendicitis had the greatest numbers of microchimeric cells in the appendix, compared with chronic inflammation or normal tissues. In addition, microchimeric frequency was higher in those with a male fetus in utero at the time of appendicectomy, compared with those with a previous male pregnancy. These findings suggested that fetal cells were present at sites of injury, had differentiated and participated in tissue repair during pregnancy.32 In addition, fetal cells appeared to respond according to the type of injury and in proportion to the amount of damage. Therefore, in response to signals produced by injured tissues or inflammation, it is possible that microchimeric fetal cells proliferate locally and differentiate into specialized cells or, alternatively, after injury fetal cells migrate from marrow to sites of damage to become involved in tissue repair.32
Go to:
PROPOSED IMPACT OF FETAL MICROCHIMERISM ON WOUND HEALING
The normal wound healing process has three phases: inflammation, tissue formation and tissue remodelling that overlap in time, and remodelling can continue for over a year. Bone marrow promotes healing and reconstitutes skin, and experimental work has already shown that whole bone marrow, as well as cultured stem cells from marrow, accelerates wound healing.47 Microchimeric fetal stem cells could be recruited from maternal bone marrow to sites of skin injury along with the endogenous stem cell population, but there is no evidence – yet, to support a beneficial effect of these primitive fetal stem cells on wound healing in the adult.
However, fetal wound healing is unique and fetal skin wounds heal rapidly without scar formation. These wound-healing properties appear to be intrinsic to fetal skin rather than a result of the fetal microenvironment and are most likely due to properties of fetal fibroblasts. There is also evidence from animal models that the state of differentiation of fetal cells is an important factor in determining scarless healing, with early gestation fetal cells healing without scar formation compared with later gestation fetal cells.48 In the adult, age-related delayed wound healing has been blamed on an altered inflammatory response, declining fibroblast function and changes in angiogenesis. There are gender differences too, with less effective skin repair mechanisms obvious in postmenopausal women. If microchimeric fetal cells are drawn to sites of skin injury, wound healing in the adult after pregnancy may become more fetal-like, given the presence of fetal cells in the adult wound. This is an exciting area of ongoing research into fetal microchimerism.
Go to:
‘GENDER MATTERS’
The pregnancy-acquired low-grade chimeric state of women could have implications for graft survival after transplantation.3 Tissues or organs taken from a woman who has been pregnant are likely to contain a mixture of her own cells and those of her children. Multiparous donors confer a higher rate of GVHD on their recipients, presumably due to the presence of additional microchimeric cell populations. Similarly, graft survival is worse in women after several pregnancies, suggesting that multiple fetal cell populations increase rejection risk. It is now acknowledged that the majority of reports of stem cell trafficking and differentiation post-transplantation in adults require re-interpretation as a Y-chromosome within female tissues could be a consequence of fetal microchimerism rather than migration of recipient stem cells.49
Go to:
WHY DO WOMEN LIVE LONGER THAN MEN?
Persisting fetal cells may also create other advantages for maternal health and influence longevity, especially if stem cells are involved. Properties of stem cells include their capacity for repeated self-renewal and their long-term multilineage potential. Little is known of these characteristics in situations where allogeneic stem cells compete with resident stem cells, as in fetal microchimerism, and it is interesting to speculate on the possible benefits to the mother of an influx of stem cells from the fetus.1,3 Epidemiological research also supports a role for pregnancy in determining longevity, and transplantation research associates cell migration and establishment in parts of the host with long-term survival of both graft and recipient. Therefore, fetal microchimerism may be a natural way for the adult female to obtain useful primitive stem cells, a mechanism to provide genetic stability and ensure her longevity.3,50
All types of human chimeras share a single biological trait: they initiate only during pregnancy.50 Human fetuses are described as ‘semi-allogeneic grafts challenging the maternal immune system’. Spontaneous chimerism is thus proposed as an evolutionally beneficial state.1,12,50 On the one hand, microchimerism occurring in human pregnancy may be designed to teach the fetus different immune mechanisms to prevent the establishment and the expansion of dangerous variants and to influence future disease susceptibility.50 On the other hand, increased fetal microchimerism is often the result of a complication during pregnancy, so this larger transfusion of fetal cells assists maternal recovery and survival after delivery.1 Pregnancy confers a lasting biological advantage on the mother.
Go to:
CONCLUSION
Fetal cells cross from the fetus into the mother's circulation during pregnancy and persist in her bone marrow for years afterwards, which implies that all parous women are chimeric. The fetal cells involved are likely to be stem cells, which pass into maternal blood in early pregnancy. While persistent fetal cells were initially thought to cause disease, animal studies now suggest that these fetal microchimeric cells are involved in the repair of damaged maternal tissues after a variety of different types of injury. Studies in human pregnancy have recently found microchimeric fetal cells present in numbers at sites of maternal tissue injury both during and decades after pregnancy.
Therefore, through pregnancy, it appears the mother gains cells that may have therapeutic potential. Fetal microchimerism contributes to the mother's ability to repair damaged or unhealthy tissue, which may be of relevance for long-term maternal health, regulation of ageing and control of longevity. Future human studies of fetal microchimerism must focus on characterizing the fetal cell type involved, resolving the debate on which stem cell type is responsible and also establishing the competitive advantage of fetal stem cells over the endogenous adult stem cell population. Determining whether microchimeric fetal cells can create functional improvement in response to maternal injury or tissue damage is key to understanding the implications of fetal microchimerism for long-term maternal health.
Go to:
ACKNOWLEDGEMENTS
Substantial contributions to the work on fetal microchimerism in lung tumours and appendicectomy specimens by Dr Hanan A Sultan MSc FRCOG and Ms Margarida Avo Santos MSc respectively, as well as by Professor Nicholas M Fisk, are acknowledged. KO'D has no conflicts of interest to declare.
Go to:
REFERENCES
- Hall JG. The importance of the fetal origins of adult disease for geneticists. Clin Genet 2007;72:67–73 [PubMed] [Google Scholar]
- Jackson L. Fetal cells and DNA in maternal blood. Prenat Diagn 2003;23:837–46 [PubMed] [Google Scholar]
- O'Donoghue K. Implications of fetal stem cell trafficking in pregnancy. Rev Gynaecol Perinatal Pract 2006;6:87–98 [Google Scholar]
- Bianchi DW. Fetal cells in the mother: from genetic diagnosis to diseases associated with fetal cell microchimerism. Eur J Obstet Gynecol Reprod Biol 2000;92:103–8 [PubMed] [Google Scholar]
- Benirschke K, Kaufmann P. Pathology of the Human Placenta. 4th edn New York: Springer-Verlag, Inc., 2000. [Google Scholar]
- Bianchi DW, Zickwolf GK, Weil GJ, Sylvester S, DeMaria MA. Male fetal progenitor cells persist in maternal blood for as long as 27 years postpartum. Proc Natl Acad Sci USA 1996;93:705–8 [PMC free article] [PubMed] [Google Scholar]
- Bazopoulou-Kyrkanidou E. Chimeric creatures in Greek mythology and reflections in science. Am J Med Genet 2001;100:66–80 [PubMed] [Google Scholar]
- Monaco AP. Chimerism in organ transplantation: conflicting experiments and clinical observations. Transplantation 2003;75:13S–16S [PubMed] [Google Scholar]
- Fast LD. Microchimerism: a lasting legacy of transfusion? Transfusion 2006;46:1856–8 [PubMed] [Google Scholar]
- Liegeois A, Gaillard MC, Ouvre E, Lewin D. Microchimerism in pregnant mice. Transplant Proc 1981;13:1250–2 [PubMed] [Google Scholar]
- Nelson JL, Furst DE, Maloney S, et al. Microchimerism and HLA-compatible relationships of pregnancy in scleroderma. Lancet 1998;351:559–62 [PubMed] [Google Scholar]
- Gleicher N, Barad DH. Gender as risk factor for autoimmune diseases. J Autoimmun 2007;28:1–6 [PubMed] [Google Scholar]
- Stevens AM. Microchimeric cells in systemic lupus erythematosus: targets or innocent bystanders? Lupus 2006;15:820–6 [PubMed] [Google Scholar]
- Artlett CM, Smith JB, Jiminez SA. Identification of Fetal DNA and Cells in Skin Lesions from Women with Systemic Sclerosis. New Engl J Med 1998;338:1186–91 [PubMed] [Google Scholar]
- Johnson KL, Nelson JL, Furst DE, et al. Fetal cell microchimerism in tissue from multiple sites in women with systemic sclerosis. Arthritis Rheum 2001;44:1848–54 [PubMed] [Google Scholar]
- Lambert N, Nelson JL. Microchimerism in autoimmune disease: more questions than answers? Autoimmun Rev 2003;2:133–9 [PubMed] [Google Scholar]
- Johnson KL, McAlindon TE, Mulcahy E, Bianchi DW. Microchimerism in a female patient with systemic lupus erythematosus. Arthritis Rheum 2001;44:2107–11 [PubMed] [Google Scholar]
- Bianchi DW, Robert E. Gross Lecture. Fetomaternal cell trafficking: a story that begins with prenatal diagnosis and may end with stem cell therapy. J Pediatr Surg 2007;42:12–8 [PubMed] [Google Scholar]
- Nelson JL. Pregnancy and microchimerism in autoimmune disease: protector or insurgent? Arthritis Rheum 2002;46:291–7 [PubMed] [Google Scholar]
- Adams KM, Lambert NC, Heimfeld S, et al. Male DNA in female donor apheresis and CD34-enriched products. Blood 2003;102:3845–7 [PubMed] [Google Scholar]
- Khosrotehrani K, Johnson KL, Guegan S, Stroh H, Bianchi DW. Natural history of fetal cell microchimerism during and following murine pregnancy. J Reprod Immunol 2005;66:1–12 [PubMed] [Google Scholar]
- O'Donoghue K, Chan J, de la Fuente J, et al. Microchimerism in female bone marrow and bone decades after fetal mesenchymal stem-cell trafficking in pregnancy. Lancet 2004;364:179–82 [PubMed] [Google Scholar]
- Khosrotehrani K, Johnson KL, Lau J, Dupuy A, Cha DH, Bianchi DW. The influence of fetal loss on the presence of fetal cell microchimerism: a systematic review. Arthritis Rheum 2003;48:3237–41 [PubMed] [Google Scholar]
- Sarkar K, Miller FW. Possible roles and determinants of microchimerism in autoimmune and other disorders. Autoimmun Rev 2004;3:454–63 [PubMed] [Google Scholar]
- Nelson JL. HLA relationships of pregnancy, microchimerism and autoimmune disease. J Reprod Immunol 2001;52:77–84 [PubMed] [Google Scholar]
- Khosrotehrani K, Bianchi DW. Multi-lineage potential of fetal cells in maternal tissue: a legacy in reverse. J Cell Sci 2005;118:1559–63 [PubMed] [Google Scholar]
- Artlett CM, O'Hanlon TP, Lopez AM, Song YW, Miller FW, Rider LG. HLA-DQA1 is not an apparent risk factor for microchimerism in patients with various autoimmune diseases and in healthy individuals. Arthritis Rheum 2003;48:2567–72 [PubMed] [Google Scholar]
- Christner PJ, Artlett CM, Conway RF, Jimenez SA. Increased numbers of microchimeric cells of fetal origin are associated with dermal fibrosis in mice following injection of vinyl chloride. Arthritis Rheum 2000;43:2598–605 [PubMed] [Google Scholar]
- Wang Y, Iwatani H, Ito T, et al. Fetal cells in mother rats contribute to the remodeling of liver and kidney after injury. Biochem Biophys Res Commun 2004;325:961–7 [PubMed] [Google Scholar]
- Imaizumi M, Pritsker A, Unger P, Davies TF. Intrathyroidal fetal microchimerism in pregnancy and postpartum. Endocrinology 2002;143:247–53 [PubMed] [Google Scholar]
- Khosrotehrani K, Johnson KL, Cha DH, Salomon RN, Bianchi DW. Transfer of fetal cells with multilineage potential to maternal tissue. JAMA 2004;292:75–80 [PubMed] [Google Scholar]
- Santos MA, O'Donoghue K, Wyatt-Ashmead J, Fisk NM. Fetal cells in the maternal appendix – a marker of inflammation or fetal tissue repair? Hum Reprod (in press 2008) [PubMed]
- Kremer Hovinga IC, Koopmans M, Baelde HJ, de Heer E, Bruijn JA, Bajema IM. Tissue chimerism in systemic lupus erythematosus is related to injury. Ann Rheum Dis 2007;66:1568–73 [PMC free article] [PubMed] [Google Scholar]
- O'Donoghue K, Sultan H, Al-Allaf F, Anderson JR, Wyatt-Ashmead J, Fisk N. Microchimeric fetal cells cluster at sites of tissue injury in lung decades after pregnancy. Reprod BioMed Online 2008;16:382–90 [PubMed] [Google Scholar]
- Johnson KL, Samura O, Nelson JL, McDonnell MdWM, Bianchi DW. Significant fetal cell microchimerism in a non-transfused woman with hepatitis C: Evidence of long-term survival and expansion. Hepatology 2002;36:1295–7 [PubMed] [Google Scholar]
- Sawaya HH, Jimenez SA, Artlett CM. Quantification of fetal microchimeric cells in clinically affected and unaffected skin of patients with systemic sclerosis. Rheumatology (Oxford) 2004;43:965–8 [PubMed] [Google Scholar]
- Srivatsa B, Srivatsa S, Johnson KL, Samura O, Lee SL, Bianchi DW. Microchimerism of presumed fetal origin in thyroid specimens from women: a case-control study. Lancet 2001;358:2034–8 [PubMed] [Google Scholar]
- Khosrotehrani K, Reyes RR, Johnson KL, et al. Fetal cells participate over time in the response to specific types of murine maternal hepatic injury. Hum Reprod 2007;22:654–61 [PubMed] [Google Scholar]
- Tan XW, Liao H, Sun L, Okabe M, Xiao ZC, Dawe GS. Fetal microchimerism in the maternal mouse brain: a novel population of fetal progenitor or stem cells able to cross the blood-brain barrier? Stem Cells 2005;23:1443–52 [PubMed] [Google Scholar]
- Huu SN, Oster M, Uzan S, Chareyre F, Aractingi S, Khosrotehrani K. Maternal neoangiogenesis during pregnancy partly derives from fetal endothelial progenitor cells. Proc Natl Acad Sci USA 2007;104:1871–6 [PMC free article] [PubMed] [Google Scholar]
- Fisk N, O'Donoghue K, Amin F, et al. Microchimeric fetal cells contribute to post-reproductive maternal tissue repair in murine and human models. Cytotherapy 2007;9:247 [Google Scholar]
- Skuladottir H, Olsen JH. Can reproductive pattern explain better survival of women with lung cancer? Acta Oncol 2006;45:47–53 [PubMed] [Google Scholar]
- Gadi VK, Nelson JL. Fetal microchimerism in women with breast cancer. Cancer Res 2007;67:9035–8 [PubMed] [Google Scholar]
- Cha D, Khosrotehrani K, Kim Y, Stroh H, Bianchi DW, Johnson KL. Cervical cancer and microchimerism. Obstet Gynecol 2003;102:774–81 [PubMed] [Google Scholar]
- Haura EB. Is repetitive wounding and bone marrow-derived stem cell mediated-repair an etiology of lung cancer development and dissemination? Med Hypotheses 2006;67(4):951–6 [PubMed] [Google Scholar]
- Aractingi S, Berkane N, Bertheau P, et al. Fetal DNA in skin of polymorphic eruptions of pregnancy. Lancet 1998;352:1898–901 [PubMed] [Google Scholar]
- Badiavas EV. The potential of bone marrow cells to orchestrate homeostasis and healing in skin. Blood Cells Mol Dis 2004;32:21–3 [PubMed] [Google Scholar]
- Longaker MT, Whitby DJ, Ferguson MW, Lorenz HP, Harrison MR, Adzick NS. Adult skin wounds in the fetal environment heal with scar formation. Ann Surg 1994;219:65–72 [PMC free article] [PubMed] [Google Scholar]
- Bianchi DW, Fisk NM. Fetomaternal cell trafficking and the stem cell debate: gender matters. JAMA 2007;297:1489–91 [PubMed] [Google Scholar]
- Rinkevich B. Human natural chimerism: an acquired character or a vestige of evolution? Hum Immunol 2001;62:651–7 [PubMed] [Google Scholar]
- Evans PC, Lambert N, Maloney S, Furst DE, Moore JM, Nelson JL. Long-term fetal microchimerism in peripheral blood mononuclear cell subsets in healthy women and women with scleroderma. Blood 1999;93:2033–7 [PubMed] [Google Scholar]
- Nelson JL. Microchimerism: expanding new horizon in human health or incidental remnant of pregnancy? Lancet 2001;358:2011–2 [PubMed] [Google Scholar]
- Aractingi S, Sibilia J, Meignin V, et al. Presence of microchimerism in labial salivary glands in systemic sclerosis but not in Sjogren's syndrome. Arthritis Rheum 2002;46:1039–43 [PubMed] [Google Scholar]
- Ichikawa N, Kotake S, Hakoda M, Kamatani N. Microchimerism in Japanese patients with systemic sclerosis. Arthritis Rheum 2001;44:1226–8 [PubMed] [Google Scholar]
- Miyashita Y, Ono M, Ono M, Ueki H. Y chromosome microchimerism in rheumatic autoimmune disease. Ann Rheum Dis 2000;59:655–6 [PMC free article] [PubMed] [Google Scholar]
- Ohtsuka T, Miyamoto Y, Yamakage A, Yamazaki S. Quantitative analysis of microchimerism in systemic sclerosis skin tissue. Arch Dermatol Res 2001;293:387–91 [PubMed] [Google Scholar]
- Selva-O'Callaghan A, Mijares-Boeckh-Behrens T, Prades EB, et al. Lack of evidence of fetal microchimerism in female Spanish patients with systemic sclerosis. Lupus 2003;12:15–20 [PubMed] [Google Scholar]
- Abbud Filho M, Pavarino-Bertelli EC, Alvarenga MP, et al. Systemic lupus erythematosus and microchimerism in autoimmunity. Transplant Proc 2002;34:2951–2 [PubMed] [Google Scholar]
- Mosca M, Curcio M, Lapi S, et al. Correlations of Y chromosome microchimerism with disease activity in patients with SLE: analysis of preliminary data. Ann Rheum Dis 2003;62:651–4 [PMC free article] [PubMed] [Google Scholar]
- Khosrotehrani K, Mery L, Aractingi S, Bianchi DW, Johnson KL. Absence of fetal cell microchimerism in cutaneous lesions of lupus erythematosus. Ann Rheum Dis 2005;64:159–60 [PMC free article] [PubMed] [Google Scholar]
- Toda I, Kuwana M, Tsubota K, Kawakami Y. Lack of evidence for an increased microchimerism in the circulation of patients with Sjogren's syndrome. Ann Rheum Dis 2001;60:248–53 [PMC free article] [PubMed] [Google Scholar]
- Carlucci F, Priori R, Valesini G. Microchimerism in Sjogren's syndrome. Rheumatology (Oxford) 2003;42:486–7 [PubMed] [Google Scholar]
- Mijares-Boeckh-Behrens T, Selva-O'Callaghan A, Solans-Laque R, Bosch-Gil JA, Vilardell-Tarres M, Balada-Prades E. Fetal microchimerism in Sjogren's syndrome. Ann Rheum Dis 2001;60:897–8 [PMC free article] [PubMed] [Google Scholar]
- Endo Y, Negishi I, Ishikawa O. Possible contribution of microchimerism to the pathogenesis of Sjogren's syndrome. Rheumatology (Oxford) 2002;41:490–5 [PubMed] [Google Scholar]
- Kuroki M, Okayama A, Nakamura S, et al. Detection of maternal-fetal microchimerism in the inflammatory lesions of patients with Sjogren's syndrome. Ann Rheum Dis 2002;61:1041–6 [PMC free article] [PubMed] [Google Scholar]
- Rubbia-Brandt L, Philippeaux MM, Chavez S, Mentha G, Borisch B, Hadengue A. FISH for Y chromosome in women with primary biliary cirrhosis: lack of evidence for leukocyte microchimerism. Hepatology 1999;30:821–2 [PubMed] [Google Scholar]
- Tanaka A, Lindor K, Gish R, et al. Fetal microchimerism alone does not contribute to the induction of primary biliary cirrhosis. Hepatology 1999;30:833–8 [PubMed] [Google Scholar]
- Invernizzi P, De Andreis C, Sirchia SM, et al. Blood fetal microchimerism in primary biliary cirrhosis. Clin Exp Immunol 2000;122:418–22 [PMC free article] [PubMed] [Google Scholar]
- Fanning PA, Jonsson JR, Clouston AD, et al. Detection of male DNA in the liver of female patients with primary biliary cirrhosis. J Hepatol 2000;33:690–5 [PubMed] [Google Scholar]
- Corpechot C, Barbu V, Chazouilleres O, Poupon R. Fetal microchimerism in primary biliary cirrhosis. J Hepatol 2000;33:696–700 [PubMed] [Google Scholar]
- Schoniger-Hekele M, Muller C, Ackermann J, et al. Lack of evidence for involvement of fetal microchimerism in pathogenesis of primary biliary cirrhosis. Dig Dis Sci 2002;47:1909–14 [PubMed] [Google Scholar]
- Klintschar M, Schwaiger P, Mannweiler S, Regauer S, Kleiber M. Evidence of fetal microchimerism in Hashimoto's thyroiditis. J Clin Endocrinol Metab 2001;86:2494–8 [PubMed] [Google Scholar]
- Renne C, Ramos Lopez E, Steimle-Grauer SA, et al. Thyroid fetal male microchimerisms in mothers with thyroid disorders: presence of Y-chromosomal immunofluorescence in thyroid-infiltrating lymphocytes is more prevalent in Hashimoto's thyroiditis and Graves' disease than in follicular adenomas. J Clin Endocrinol Metab 2004;89:5810–4 [PubMed] [Google Scholar]
- Klintschar M, Immel UD, Kehlen A, et al. Fetal microchimerism in Hashimoto's thyroiditis: a quantitative approach. Eur J Endocrinol 2006;154:237–41 [PubMed] [Google Scholar]
- Ando T, Imaizumi M, Graves PN, Unger P, Davies TF. Intrathyroidal fetal microchimerism in Graves' disease. J Clin Endocrinol Metab 2002;87:3315–20 [PubMed] [Google Scholar]
- Lombardi T, Philippeaux MM, Hadengue A, Samson J, Borisch B, Rubbia-Brandt L. Absence of leukocyte microchimerism in oral lichen planus (OLP): an in situ hybridisation study. J Oral Pathol Med 2001;30:398–401 [PubMed] [Google Scholar]
- Weger W, Bauer M, Odell E, et al. Role of microchimerism in the pathogenesis of oral lichen planus. Exp Dermatol 2006;15:125–9 [PubMed] [Google Scholar]
- Regauer S, Schwaiger P, Liegl B, Klintschar M. Fetal microchimerism is common in normal and diseased vulvar skin. J Invest Dermatol 2004;122:1059–60 [PubMed] [Google Scholar]
- Bauer M, Weger W, Orescovic I, et al. Fetal microchimerism is not involved in the pathogenesis of lichen sclerosus of the vulva. Prenat Diagn 2006;26:175–8 [PubMed] [Google Scholar]
- Guettier C, Sebagh M, Buard J, et al. Male cell microchimerism in normal and diseased female livers from fetal life to adulthood. Hepatology 2005;42:35–43 [PubMed] [Google Scholar]
- Bustos ML, Frias S, Ramos S, et al. Local and circulating microchimerism is associated with hypersensitivity pneumonitis. Am J Respir Crit Care Med 2007;176:90–5 [PubMed] [Google Scholar]
https://pubmed.ncbi.nlm.nih.gov/19262088/
सर्वतीरथमयी माता सर्वदेवमय: पिता
मातरम पितरन तस्मात सर्व्यतनें पूजयते।
अर्थ
माता समस्त तीरथों के समान है तथा पिता समस्त देवों के समान पूजनीय होते हैं। अतः मनुष्य का यह परम कर्तव्य है कि वह माता पिता का आदर सत्कार करे।
——
माता भूमि पुत्रोंई’ पृथित्या
पृथिवी मेरी माता है और मैं उसकी पुत्री हूँ।
नास्ति मत्रिसमI छाया
नास्ति मत्रिसमI गति:
नास्ति मत्रिसमI त्राण:
नास्ति मत्रिसमI प्रपा।
अर्थ
माता के समान कोई छाया नहीं, आश्रय नहीं, सुरक्षा नहीं। माता के समान इस दुनिया मैं कोई जीवन दाता नहीं।

https://online.fliphtml5.com/biiwr/wkbj/#p=4